Lionel Brisseau , Andrei Chiveri , Denis Lebel , Jean-François BussièresABSTRACT
Background:
In 2004, the US Food and Drug Administration issued a new rule requiring most prescription and some over-the-counter pharmaceutical products to carry bar codes down to the level of individual doses, with the intent of reducing the number of medication errors. Despite these regulatory changes in the United States, Health Canada has not yet adopted any mandatory bar-coding of drugs.
Objective:
To evaluate the feasibility of using commercial bar codes for receipt and preparation of drug products and to evaluate the readability of the bar codes printed on various levels of drug packaging.
Methods:
This cross-sectional observational pilot study was conducted in the Pharmacy Department of a Canadian mother–child university hospital centre in July 2010. For the purposes of the study, research drugs and cytotoxic drugs in various storage areas, as well as locally compounded medications with bar codes generated in house, were excluded. For all other drug products, the presence or absence of bar codes was documented for each level of packaging, along with the trade and generic names, content (i.e., drug product), quantity of doses or level of packaging, therapeutic class (if applicable), type of bar code (1- or 2-dimensional symbology), alphanumeric value contained in the bar code, standard of reference used to generate the alphanumeric value (Universal Product Code [UPC], Global Trade Item Number [GTIN], or unknown), and readability of the bar codes by 2 scanners.
Results:
Only 33 (1.9%) of the 1734 products evaluated had no bar codes on any level of packaging. Of the 2875 levels of packaging evaluated, 2021 (70.3%) had at least one bar code. Of the 2384 bar codes evaluated, 2353 (98.7%) were linear (1-dimensional) and 31 (1.3%) were 2-dimensional. Well over three-quarters (2112 or 88.6%) of the evaluated bar codes were readable by at least 1 of the 2 scanners used in the study.
Conclusions:
On the basis of these results, bar-coding could be used for receipt of 80.9% of the drug products at this Canadian hospital and for the preparation and dispensing of 70.1% of the products.
KEYWORDS: bar-coding , pharmaceutical products , packaging
RÉSUMÉ
Contexte :
En 2004, la US Food and Drug Administration a mis de l’avant un nouveau règlement exigeant que la plupart des produits pharmaceutiques d’ordonnance et certains produits pharmaceutiques en vente libre portent des codes-barres, y compris les emballages unitaires, dans le but de réduire le nombre d’erreurs de médication. Malgré cette nouvelle réglementation aux États-Unis, Santé Canada n’a pas encore adopté un tel règlement au pays.
Objectif :
Évaluer la faisabilité de l’utilisation de codes-barres commerciaux pour la réception et la préparation des produits pharmaceutiques et la lisibilité des codes-barres imprimés sur différents niveaux de conditionnement.
Méthodes :
Il s’agit d’une étude pilote d’observation transversale menée dans le service de pharmacie d’un centre hospitalier universitaire mère-enfant canadien en juillet 2010. Aux fins de l’étude, les médicaments de recherche et les médicaments cytotoxiques dans diverses aires d’entreposage, de même que les médicaments préparés sur place, portant des codes-barres maison, ont été exclus. Pour tous les autres produits pharmaceutiques, la présence ou l’absence de codes-barres a été constatée pour chaque niveau de conditionnement, de même que les dénominations commerciale et commune, le contenu (c.-à-d. le produit pharmaceutique), la quantité de doses ou le niveau de conditionnement, la classe thérapeutique (le cas échéant), le type de code-barres (unidimensionnel ou bidimensionnel), la valeur alphanumérique du code-barres, la norme de référence utilisée pour générer la valeur alphanumérique (code universel des produits [CUP], code article international [ GTIN ] ou inconnue) et la lisibilité des codes-barres au moyen de deux lecteurs différents.
Résultats :
Seulement 33 (1,9 %) des 1734 produits évalués ne portaient pas de code-barres peu importe le niveau de conditionnement. Des 2875 niveaux de conditionnement évalués, 2021 (70,3 %) portaient au moins un code-barres. Des 2384 codes-barres évalués, 2353 (98,7 %) étaient linéaires (unidimensionnels) et 31 (1,3 %) étaient bidimensionnels. Bien au-delà des trois-quarts (2112 ou 88,6 %) des codes-barres évalués ont pu être décodés par au moins un des deux lecteurs utilisés.
Conclusions :
Ces résultats montrent que les codes-barres pourraient être utilisés pour la réception de 80,9 % des produits pharmaceutiques dans cet établissement de santé canadien ainsi que pour la préparation et la distribution de 70,1 % des produits pharmaceutiques.
MOTS CLÉS: code-barres , produits pharmaceutiques , conditionnement
[Traduction par l’éditeur]
In 2004, the US Food and Drug Administration (FDA) issued a new rule requiring most prescription and some over-the-counter pharmaceutical products to carry bar codes down to the level of individual doses.1 The new rule was intended to reduce the number of medication errors associated with drug products. However, the FDA did not require manufacturers to include the expiration date and lot number in their bar-coded product numbers.
In early 2010, the American Society of Health-System Pharmacists (ASHP) published a draft statement on bar-code verification during inventory, preparation, and dispensing of medications.2 In its review of the literature, the ASHP found that although “initial estimates of the contribution of pharmacy dispensing errors to the overall medication errors were quite low . . . reports have suggested that adding bar coding to the pharmacy dispensing process can significantly reduce opportunities for medication errors at the bedside and reduce the occurrence of potential adverse drug reactions.” ASHP insisted that “as with other bar-code technology implementations, pharmacy-based bar-code scanning systems will only be beneficial if appropriately deployed.”
Despite the US regulatory changes, Health Canada has not yet adopted any mandatory bar-coding of drugs. In January 2008, an invitational stakeholder round table, cochaired by the Institute for Safe Medication Practices Canada (ISMP Canada) and the Canadian Patient Safety Institute (CPSI), was convened in Ottawa to discuss and seek consensus from pharmaceutical manufacturers on voluntary guidelines for the use of bar codes to label medications at the unit-of-use packaging level. Also in 2010, ISMP Canada and CPSI jointly endorsed adoption of the GS1 global standard for automated identification of pharmaceutical products in Canada.3 ISMP Canada indicated that the “adoption of a Canadian standard for automated identification of medications will give integrated healthcare solution providers the necessary expectations about future practice to allow them to develop automated methods for identifying products and checking the safety of specific dosages within their proprietary patient care software modules.”3 Readers may consult the ISMP Canada website to learn about this multiphase project, which includes joint technical requirements for Canadian pharmaceuticals, bar-code components and symbologies, elements to be used in product databases, medications in the categories to be bar-coded, and bar-code placement on various packaging levels.4
In the Canadian market, the pharmaceutical industry uses Universal Product Code (UPC) numbers and Global Trade Item Numbers (GTINs), both of which are derived from the GS1 standard. The UPC is a 12-digit number consisting of a 6-digit component identifying the manufacturer, followed by a 5-digit component identifying the product and a checksum digit. The GTIN is a 14-digit number consisting of a 2-digit component for the level of packaging, a 6-digit component identifying the manufacturer, a 5-digit component identifying the product, and a checksum digit. However, according to the GS1 data bar symbology, a 16-digit number is required to represent a GTIN, with the addition of a 2-digit application identifier indicating use of a GTIN in a specific bar code. The GTIN is compatible with existing standards such as the UPC and usually does not place any additional requirements on scanning hardware.5
The Centre Hospitalier Universitaire Sainte-Justine (CHU Sainte-Justine) in Montréal, Quebec, has been using bar codes for many years, mainly for inventory management, replenishment of ward stock, and packaging of unit doses for oral administration. Use of bar codes helps to confirm the identity of a drug product and to verify correspondence between the institutional purchase order and the product received from the manufacturer or wholesaler. The use of bar codes can also help to confirm the identity of a product when medications are prepared for administration to patients (i.e., ad hoc preparation or delivery according to the distribution mode of the medication-use system).
Over the past few years, the authors have explored the concept of numeric identity for drug products, whereby all relevant information for a given medication (e.g., drug numbers, including applicable bar-code numbers; description and characteristics of the drug; images; pronunciation of the drug name) is entered into a single database that can support the various software programs used throughout the drug distribution process.6 Ideally, a Canadian drug product database that includes bar-code data should be available to all pharmacists to support a safe medication-use system in all hospital settings.7
The aim of this pilot study was to evaluate the feasibility of using commercial bar codes for the receipt and preparation of drug products and to evaluate the readability of commercial bar codes printed on various levels of drug packaging.
This cross-sectional observational study was conducted in July 2010 in the Pharmacy Department of the CHU Sainte-Justine, a university-affiliated mother–child hospital centre. The Pharmacy Department deals with more than 150 suppliers, including wholesalers, for more than 3200 products. Over the course of the 2009/2010 financial year, the stock management team generated 3459 purchase orders for a total of 22 993 line items, where each line item specifies an order of a given quantity of a particular product. In the same financial year, medication expenses totalled $17 195 938 for a total of 2 692 130 dispensed doses.
This study focused on all levels of commercial drug packaging available in the central storage area of the Pharmacy Department at CHU Sainte-Justine, including the vault of controlled substances. Any given product could have one or more levels of packaging, including pallets, crates, boxes, and individual units. Research drugs and dangerous cytotoxic drugs in various storage areas and locally compounded medications with bar codes generated in house were excluded.
The presence or absence of bar codes was documented for each level of packaging available in the Pharmacy Department’s central storage area. In addition, for each level of packaging, the trade and generic names, content (i.e., drug product), quantity of doses or level of packaging, therapeutic class (if applicable), type of bar code (1- or 2-dimensional symbology), alphanumeric value contained in the bar code, standard of reference used to generate the alphanumeric value (UPC, GTIN, or unknown), and readability of the bar codes by a table scanner and a hand-held scanner (models LS9208 and LS4208, respectively; Motorola, Schaumburg, Illinois; capable of reading only 1-dimensional bar codes) were documented. A bar code was considered readable if the alphanumeric value could be read by one or both of the scanners without any reconfiguration.
The wholesaler for CHU Sainte-Justine (Mckesson Canada) was asked to supply a file of all products (i.e., drugs) that could be sold to Quebec hospitals in 2010. Examination of the database revealed that the wholesaler enters bar codes for some levels of packaging, but it was not known to what extent such information is collected in the database. The local results obtained for CHU Sainte-Justine were compared with the wholesaler’s database with respect to presence or absence of bar codes by product and by level of packaging. No statistical analyses were performed.
The feasibility of using commercial bar codes for receipt of ordered products and during preparation of medications for dispensing was evaluated, taking into account the specific products and quantities dispensed in 2009/2010.
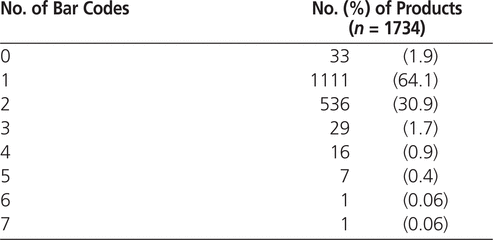
A total of 1734 products from 167 manufacturers were evaluated, including 1550 (89.4%) with a Canadian identification number (Drug Identification Number [DIN] or Natural Product Number [NPN]). The products without a Canadian identification number were chemical compounds used in pharmaceutical compounding or products from other countries that were imported through Health Canada’s Special Access Programme. Some of these products without a Canadian identification number had bar codes. Only 33 (1.9%) of the products evaluated had no bar codes on any level of packaging (Table 1). Of the 2875 levels of packaging evaluated, 2021 (70.3%) had at least one bar code (Table 2). Of the 2384 individual bar codes evaluated, 2353 (98.7%) were linear (1-dimensional) and 31 (1.3%) were 2-dimensional.
Table 1.
Number of Bar Codes per Product, as Printed on All Packaging Levels

Table 2.
Number of Bar Codes Printed on Each Individual Packaging Level

Overall, 2112 (88.6%) of the 2384 bar codes evaluated were readable (as defined within the context of this study) by at least 1 of the 2 scanners, and 1986 (83.3%) were readable by both scanners. Of the 1640 bar codes with a UPC value, 1630 (99.4%) were readable by the table scanner and 1612 (98.3%) were readable by the portable scanner. Of the 212 bar codes with a GTIN value, 29 (13.7%) were readable by the table scanner and 59 (27.8%) were readable by the portable scanner. The scanners used in this study had more difficulty reading bar codes printed on small vials or ampoules.
For assessing the feasibility of using bar codes for receipt of stock and preparation and dispensing of medications, it was assumed that all bar codes would be readable (i.e., after procurement of new scanners or appropriate configuration of existing scanners). It was determined that CHU Sainte-Justine would be able to use the existing bar code for the receipt of 1644 (94.8%) of its 1734 products and for the preparation and dispensing of 1399 (80.7%) of these products. The presence of a bar code on the highest level of packaging was considered in evaluating use of bar codes during receipt of stock, and the presence of a bar code on the lowest level of packaging was considered in evaluating use of bar codes for preparation and dispensing of medication. When the proportion of usable bar codes was weighted according to quantity of drug product dispensed (with 807 of the 1734 products having an annual consumption of at least 100 units), it was determined that existing bar codes could be used for receipt of 80.9% of the products and for preparation and dispensing of 70.1% of the products.
The number of bar codes on all packaging levels of each individual product ranged from 0 to 7 (Table 1). The presence of multiple bar codes for a single product may present difficulties in terms of ensuring that the correct bar code is scanned during various drug distribution processes. The problem of multiple bar codes for a single product may be an issue even if only one level of packaging is considered, as 2 or more bar codes were present on an individual packaging level for 451 (15.7%) of the 2875 packaging levels evaluated (Table 2).
By requiring that bar codes be printed down to the smallest dose unit administered at the patient’s bedside, the FDA has indirectly contributed to several studies on the effect of bar codes on the medication-use system in pharmacy departments8,9 and health care units.10–12 As a result, many health care professionals think that using bar codes can help to reduce, though not eliminate, medication errors.13,14 Notably, emergence of the concept of human factors engineering may contribute to more effective management of the introduction and optimal use of bar codes in the medication-use system.15
The 2009–2010 survey of hospital pharmacy in Canada found that 49% of health care institutions used bar codes (72% of university hospitals and 40% of nonuniversity hospitals).16 Of the 78 health care institutions reporting that they used bar codes, the most common uses were for verifying the stocking of automated repackaging machines (69%), verifying the stocking of automated dispensing cabinets (50%), managing inventory (45%), verifying drug selection before dispensing from the pharmacy (33%), and verifying the stocking of unit-dose bins (26%). Very few respondents reported using bar codes for activities directly related to identifying patients (5 respondents), selecting drugs (6 respondents), or identifying staff members (3 respondents) before administration of a medication or for transfer of patient-specific data and/or medications to electronic pumps (8 respondents). Importantly, these survey data did not take into account the presence or absence of commercial bar codes, and they included use of locally generated bar codes (with robots, baggers, or pharmacy software programs). Similarly, reported use of bar codes for a given task gives no indication as to the volume of bar-coded products that are scanned.
Various factors may explain the lengthy delay in adopting bar-code technologies in hospital settings, for example, the costs of acquiring and implementing hardware and software, the human resources required to manage the data, the lack of standards and uniformity in commercial bar codes, and the absence of a Canadian database for bar-code identification of drugs.
No data are available concerning the presence of bar codes for the various levels of commercial drug packaging available in Canada. This pilot project revealed that more than 98% of the products available in the central storage area of the CHU Sainte-Justine in summer 2010 had at least one bar code on at least one of the packaging levels evaluated. Nevertheless, only 70.3% of all levels of packaging evaluated had at least one bar code.
The recommendations of the Canadian Pharmaceutical Bar Coding Project, including use of the GS1 standard, aim to identify all levels of drug packaging, thus making it possible to read relevant bar codes at every step of the medication-use system, from supplier to bedside administration. In this pilot study, nearly one-third of the levels of packaging had no bar codes, but 94.8% of the products had at least one level of packaging with bar codes that could be read when stock was received at the hospital from the supplier for storage, and 80.7% of the products had bar codes that could be used during ad hoc preparation of doses in the Pharmacy Department. Therefore, there is no reason for further delay in the use of bar codes in hospital practice.
The Canadian Pharmaceutical Bar Coding Project considers 2-dimensional bar codes as an opportunity for the future, because they allow display of a complete set of information about each drug product, including GTIN, batch number, and expiry date. However, in this study, only 1.3% of the bar codes evaluated were 2-dimensional. The FDA does not require the use of 2-dimensional bar codes because of the large number of 1-dimensional scanners already in use and the unconvincing cost–benefit ratio associated with adding batch numbers and expiry dates.1 Hopefully, the nonadoption of 2-dimensionality will not represent a missed opportunity as the use of bar codes becomes more widespread in hospital settings.
The bar codes printed on commercial drug packaging can usually be read by a scanner and used for inventory and other purposes. In the United States, those that are not readable can be reported to an ASHP problem-tracking website.17 It is hoped that a similar initiative will soon be available in Canada.
Given the current status of commercial bar-coding and the findings of this pilot project, the question arises of whether Canadian hospitals can proceed with implementing bar-code technology within the framework of the medication-use system. The authors of the current study believe that the answer to this question is “yes”, but the following elements must be taken into consideration. The CHU Sainte-Justine Pharmacy Department delivered a total of 2 692 130 doses in 2009/2010. A subanalysis focusing on 1 525 252 doses for the 807 products with at least 100 doses administered per year confirmed that at least 1 320 700 (86.6%) doses, from 566 (70.1%) products, were associated with a bar code that could be read. However, safe use of bar codes at different steps of the medication-use system relies on access to a Canadian drug database. Such a database should also include product descriptions, levels of packaging, applicable bar codes, and corresponding images. In addition, hospitals must consider their own internal bar-code management system when the products are repackaged.18 Ideally, local bar-coding should respect both the GS1 standard and the standards for local assignment of bar codes.19
This pilot study had some limitations. The selection of products for evaluation was based on the drug inventory available at the CHU Sainte-Justine during summer 2010, which is not necessarily representative of what would be found in other adult health care institutions. The selection excluded products used in oncology and research, as well as locally compounded products. Furthermore, a product may have several levels of packaging with bar codes that were not considered in this analysis. However, a complementary analysis conducted on a subset of the wholesaler’s database indicated a similar proportion of products and levels of packaging with bar codes. Finally, the study relied on 2 scanners set with the manufacturer’s original configuration. Although the scanners used were representative of widely available standard models, other scanners may offer different reading capabilities. Also, the configuration of bar code readers can be adjusted for optimal reading capability.
This observational pilot study focused on 1734 products used in a Canadian hospital, of which only 1.9% had no bar codes on any level of packaging. Of the 2875 levels of packaging evaluated, 70.3% had at least one bar code. When the proportion of useable bar codes was weighted according to the quantities of each product consumed, it was determined that commercial bar codes could be used for receipt of 80.9% of drug products and for preparation and dispensing of 70.1%.
1.
Bar code label requirement for human drug products and biological products: a rule by the Food and Drug Administration on 02/26/2004.
Federal Register
2004 [cited 2011 Aug 15];69:9120–9171. Available from: www.federalregister.gov/articles/2004/02/26/04-4249/bar-code-label-requirement-for-human-drug-products-and-biological-products#p-3
2. ASHP statement on bar-code verification during inventory, preparation, and dispensing of medications. Bethesda (MD): American Society of Health-System Pharmacists; 2010 [cited 2010 Oct 30]. Available from: www.ashp.org/DocLibrary/BestPractices/AutoITStBCVerif.aspx
3.
Canadian Pharmaceutical Bar Coding Project: joint technical statement on pharmaceutical automated identification and product database requirements
. Toronto (ON): Institute for Safe Medication Practices Canada; 2010 [cited 2010 Apr 30]. Available from: www.ismp-canada.org/barcoding/download/CanPharmBarcode_JointTechnicalStatement.pdf

4.
Canadian Pharmaceutical Bar Coding Project [website]. Toronto (ON): Institute for Safe Medication Practices Canada; [cited 2010 Oct 30]. Available from: www.ismp-canada.org/barcoding/index.htm
5. What is GTIN? Chicago (IL): Bar Code Graphics Inc; [cited 2010 May 6]. Available from: www.gtin.info/
6. Bussières JF, Lebel D, Voytenko S, Vaquer G. Développement d’un concept et d’un processus de gestion de l’identité numérique d’un produit en établissement de santé. Can J Hosp Pharm 2009;62(5):406–414.
7. Lebel D, Vaquer G, Forest JM, Bussières JF. Utilisation d’une banque de données photographiques consultées par code-barres pour assister la préparation de seringues orales. Pharm hosp 2009;44(3):114–124.
8.
Poon EG, Cina JL, Churchill W, Patel N, Featherstone E, Rothschild JM, et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy.
Ann Intern Med
2006;145(6):426–434.
9.
Larrabee S, Brown MM. Recognizing the institutional benefits of bar-code point-of-care technology.
Jt Comm J Qual Saf
2003;29(7):345–353.
10.
Poon EG, Keohane CA, Yoon CS, Ditmore M, Bane A, Levtzion-Korach O, et al. Effect of bar-code technology on the safety of medication administration.
N Engl J Med
2010;362(18):1698–1707.
11.
DeYoung JL, Vanderkooi ME, Barletta JF. Effect of bar-code-assisted medication administration on medication error rates in an adult medical intensive care unit.
Am J Health Syst Pharm
2009;66(12):1110–1115.
12.
Helmons PJ, Wargel LN, Daniels CE. Effect of bar-code-assisted medication administration on medication administration errors and accuracy in multiple patient care areas.
Am J Health Syst Pharm
2009; 66(13):1202–1210.
13.
Bar-code/eMAR combo reduces errors.
Healthcare Benchmarks Qual Improv
2010;17(9):100–102.
14.
O’Malley P. Think bar-code medication administration eliminates adverse drug events? Think again!
Clin Nurse Spec
2008;22(6):269–270.
15.
Escoto KH, Hallock M, Wagner J, Karsh BT. Using variance analysis to detect hazards in a bar-code-assisted medication preparation process.
Jt Comm J Qual Saf
2004;30(11):622–628.
16. Macgregor P. Technology. In: Babich M, Bornstein C, Bussières JF, Hall K, Harding J, Lefebvre P, et al., editors. Hospital pharmacy in Canada 2009/2010 report . Eli Lilly; 2010 [cited 2011 Jun 1]. Available from: www.lillyhospitalsurvey.ca/hpc2/content/2010_report/chapter_f%20.pdf
17. ASHP drug product bar-code reporting problem center [website]. Bethesda (MD): American Society of Health-System Pharmacists; [cited 2010 Oct 30]. Available from: www.ashp.org/Import/MEMBERCENTER/Sections/SectionofPharmacyInformaticsandTechnology/Resources/BarCode.aspx
18.
Pederson CA, Dumpper KF. ASHP national survey on informatics: assessment of the adoption and use of pharmacy informatics in U.S. hospitals.
Am J Health Syst Pharm
2008;65(23):2244–6224.
19. Lebel D, Bussières JF. Are you GS1-compliant? One hospital pharmacy’s experience [letter]. Can J Hosp Pharm 2010;63(4):333–334.
Canadian Journal of Hospital Pharmacy , VOLUME 64 , NUMBER 4 , July-August 2011