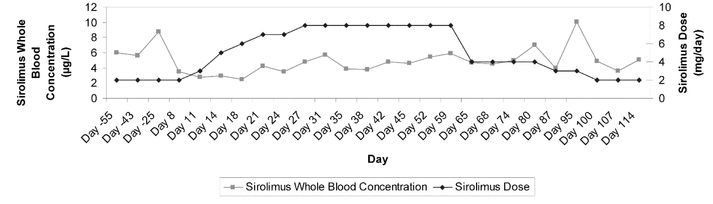
Figure 1. Summary of measured sirolimus levels and dosage adjustments for a patient with seizures. Horizontal axis (time) is not to scale.
Duane Bates , Kelly W Burak , Carla S Coffin , Tammy Ying , Echo-Marie Enns
Sirolimus is used primarily to prevent rejection of solid organ grafts. This drug is a substrate for hepatic and intestinal cytochrome P450 3A4 isozyme (CYP3A4) and P-glycoprotein and is therefore susceptible to many drug interactions.1,2 Phenytoin is a strong inducer of CYP2C9, CYP2C19, and CYP3A4, so it may increase the hepatic metabolism of many medications.3 A case of phenytoin-induced decline in sirolimus levels is reported here.
A 68-year-old man was admitted to hospital with new-onset seizures and left-sided hip pain.* His medical history included liver transplant (13 years before this admission) for alcohol-related end-stage liver disease, as well as chronic kidney disease, left posterior cerebral artery stroke with residual right-sided weakness, myocardial infarction (3 months before this admission), type 2 diabetes mellitus, hypothyroidism, glaucoma, and gastroesophageal reflux disease. Because of chronic renal failure related to the use of cyclosporine, his immunosuppression therapy had been converted to sirolimus 9 years before this admission.
The patient’s medications before admission were sirolimus 2 mg daily (for which the dose had not been changed in the past 5 years), acetylsalicylic acid 81 mg daily, clopidogrel 75 mg daily, repaglinide 2 mg 3 times daily, long-acting insulin 4–8 units at bedtime, pantoprazole 40 mg daily, metoprolol 25 mg twice daily, ezetimibe 10 mg daily, levothyroxine 50 μg daily, calcium carbonate 1250 mg daily, vitamin D 400 units daily, and dorzolamide–timolol (20 and 5 mg/mL) 1 drop in each eye daily. The median whole-blood level of sirolimus for the 6 months before admission had been 5.6 μg/L (range 4.5–13.5 μg/L), which was within the target range of 4–7 μg/L. The only recent changes in medication were initiation of clopidogrel, metoprolol, and ezetimibe after the myocardial infarction. The patient denied use of any herbal or over-the-counter medications, did not drink alcohol, and had no known drug allergies. He weighed 73.3 kg at the time of admission.
On physical examination, the patient was alert and was oriented to person, place, and time. Musculoskeletal examination revealed pain over both the lateral aspect of the left hip and the greater trochanter region. Neurological examination revealed poor memory and ataxic gait, accompanied by upper-and lower-extremity weakness. The results of head and neck, respiratory, and cardiovascular examinations were unremarkable. The patient was hemodynamically stable and afebrile.
At the time of admission, the patient’s serum creatinine level was 245 μmol/L (normal range 50–120 μmol/L), and the estimated creatinine clearance was 26 mL/min. Liver function tests showed total bilirubin 2 μmol/L (normal range 0–24 μmol/L), alkaline phosphatase 65 units/L (normal range 30–145 units/L), alanine aminotransferase 6 units/L (normal range 1–60 units/L), γ-glutamyltransferase (GGT) 23 units/L (normal range 11–63 units/L), international normalized ratio 1 (normal range 0.9–1.1), and partial thromboplastin time 29.4 s (normal range 28.1–41 s). A complete blood cell count revealed hemoglobin 98 g/L (normal range 137–180 g/L), mean corpuscular volume 80 fL (normal range 82–100 fL), platelets 311 × 109/L (normal range 150 × 109/L to 400 × 109/L), and white blood cells 9.5 × 109/L (normal range 4 × 109/L to 11 × 109/L). Serum glucose (random) was 7.9 mmol/L (normal range 3.4–11.1 mmol/L), and all electrolytes were within normal ranges.
Radiography of the left hip showed no fracture or post-traumatic deformity of the pelvis or hip. Computed tomography 6 days before admission, after a fall at home, revealed the old left posterior cerebral artery infarct, with no acute intracranial abnormalities. On consultation, the neurology service suggested that the patient had simple focal seizures progressing to generalized tonic–clonic seizures. Initiation of carbamazepine 200 mg bid was recommended, to be increased to 400 mg bid after 48 h. If there were any further seizures, phenytoin was to be added to the carbamazepine therapy.
Doppler ultrasonography of the carotid arteries on day 3 of the admission indicated no significant narrowing. On day 5, the patient experienced another tonic–clonic seizure and was given phenytoin 500 mg IV loading dose, followed by 100 mg IV q8h. By day 6, the patient was drowsy and confused, his ataxia was worsening, and his carbamazepine level was 65 μmol/L (normal range 20–50 μmol/L). The pharmacist suggested changing the anticonvulsant therapy to levetiracetam, because both carbamazepine and phenytoin may increase the metabolism of sirolimus, which could lead to subtherapeutic concentrations and potential rejection of the graft. The patient experienced further seizure activity, displaying tonic–clonic and choreiform movements of the upper extremities. The neurology service recommended continuing phenytoin 300 mg daily. In addition, the carbamazepine dose was tapered by 50% every 4 days and then stopped. Magnetic resonance imaging of the brain suggested infarcts of the left pons and a large left-sided infarct in the territory of the posterior cerebral artery, involving the temporal and occipital lobes. Electroencephalography showed intermittent interictal epileptiform discharges arising from the left posterior temporal–parietal–occipital region, in keeping with partial and/or secondary generalized seizures.
On day 8 of the admission, a sirolimus trough level was measured at 3.5 μg/L, and the dose was increased to 3 mg daily, as suggested by the liver transplant team (see Figure 1 for subsequent sirolimus levels and dosage adjustments). On day 14, the phenytoin level, corrected for albumin, was 52.1 μmol/L (normal range 40–80 μmol/L). On day 16, the carbamazepine was discontinued, and on day 21, sirolimus was 4.2 μg/L and phenytoin was 42.1 μmol/L. The patient continued to experience focal seizures of the left arm every 2 to 3 days, so on day 22, the phenytoin dose was increased to 350 mg daily. On day 24, the sirolimus level had again declined to 3.5 μg/L, and the dose was further increased to 8 mg daily. On day 27, the sirolimus level had returned to within the target range, at 4.8 μg/L. The new maintenance dose of sirolimus (8 mg daily) was well tolerated: the patient did not experience any adverse effects during his hospital stay, despite a 4-fold increase in dosage. Total bilirubin, alkaline phosphatase, and alanine aminotransferase all remained within their normal ranges, and the patient displayed no evidence of graft rejection. However, the level of GGT increased by about 10 times from baseline, secondary to the phenytoin therapy.
|
|
||
|
Figure 1. Summary of measured sirolimus levels and dosage adjustments for a patient with seizures. Horizontal axis (time) is not to scale. |
||
The patient continued to have focal seizures involving his left arm, and on day 54 the phenytoin dose was increased to 500 mg daily, despite a corrected phenytoin level of 47.4 μmol/L. On day 56, the neurology service recommended that the phenytoin dose be decreased to 400 mg daily, with a plan for complete discontinuation, and levetiracetam 500 mg twice daily be initiated (the latter drug is known to have negligible drug interactions). On day 59, the patient’s sirolimus level was 5.9 μg/L with continued administration of sirolimus 8 mg daily. On day 60, phenytoin was discontinued without tapering, and the levetiracetam dose was increased to 750 mg twice daily. It was not known how long the phenytoin-induced enzyme induction would persist after discontinuation; therefore, on day 62, the sirolimus dose was initially decreased to 4 mg daily. On day 80, the patient’s sirolimus level was 7 μg/L, and the dose was decreased to 3 mg daily. On day 86, the dose of levetiracetam was increased to 1000 mg twice daily. On day 87, the sirolimus level was 4 μg/L. The following day, the patient was discharged from hospital. On day 95, the sirolimus level was 10.1 μg/L, and the dose was decreased to the preadmission dose of 2 mg daily. The discontinuation of phenytoin allowed for a reduction in the sirolimus dose, which paralleled a fall in GGT to baseline (see Figure 2). On day 114, the patient was continuing sirolimus 2 mg daily, with stable function of the graft. The patient reported decreased frequency of seizures with the levetiracetam therapy.
|
|
||
|
Figure 2. Correlation among γ–glutamyltransferase (GGT), the course of phenytoin therapy, and the dose of sirolimus. Horizontal axis (time) is not to scale. |
||
The literature was searched for any publications describing an interaction between phenytoin and sirolimus. The search terms “sirolimus” and “phenytoin” were used to search PubMed, OVID, Embase, International Pharmaceutical Abstracts, and Reactions Weekly, from January 1948 to January 2011. Two case reports, summarized below, were identified.
The first report described a 62-year-old woman originally presenting with autoimmune hepatitis and primary biliary cirrhosis, who underwent orthotopic liver transplant.4 The patient was treated with tacrolimus-based immunosuppression. About 1 week after the transplant, a seizure disorder developed, with altered mental status and decreased level of consciousness. At the time of this neurological deterioration, phenytoin 200 mg daily was initiated (loading dose not reported), and tacrolimus was replaced by cyclosporine 200 mg daily. The cyclosporine was subsequently discontinued because of a lack of improvement in neurological status, and the patient was started on sirolimus 5 mg daily. Trough plasma phenytoin level was 21.8 μmol/L (but the authors did not report the day on which the sample was drawn). The sirolimus concentration remained below 5 μg/L, which led to an escalation of the sirolimus dose, to 15 mg daily, over a period of 15 days. Despite the increase in dose, the level of sirolimus remained subtherapeutic. Within 10 days of discontinuation of phenytoin, the sirolimus level increased to 15 to 27 μg/L, and the sirolimus dose was decreased to 10 mg daily. No further sirolimus levels or signs of rejection were reported.
In the second case, an 11-year-old girl with chronic renal failure secondary to Henoch-Schönlein nephritis underwent kidney transplant.5 Her immunosuppression regimen consisted of cyclosporine, mycophenolate mofetil, and prednisolone. On day 7 after the transplant, she had focal seizures which progressed to status epilepticus. The cyclosporine trough level was 175 nmol/L (target 150–300 nmol/L). Phenytoin was started, but the loading and maintenance doses were not reported. The cyclosporine was then discontinued, and sirolimus 0.06 mg/kg daily was started (day of initiation not reported), with target whole-blood trough level of 10–20 μg/L. On day 20, the phenytoin level was 55 μmol/L (but the authors did not report whether phenytoin measurements were corrected for albumin), and the sirolimus level was less than 5 μg/L. On day 27, the phenytoin level was 80 μmol/L, and the sirolimus level was less than 5 μg/L. Over the previous 3 weeks, the patient had had 2 episodes of biopsy-proven acute rejection, both of which were responsive to a 3-day course of methylprednisolone. There was no documentation of any change to the dose of sirolimus. On day 34, the phenytoin was discontinued (phenytoin level 39 μmol/L), and tacrolimus was added to the patient’s regimen; however, tacrolimus levels were not reported. On day 38, the patient was discharged; at that time, her serum creatinine level was within the normal range. On day 41, the sirolimus level had risen to 22 μg/L, and by day 48, it had risen further, to 39 μg/L. On day 70, the sirolimus level was 43 μg/L, at which time the dose was reduced (although the specific dose reduction was not reported), and follow-up sirolimus levels on days 79 and 83 were within the target range of 10 to 20 μg/L.
These 3 cases suggest that sirolimus levels may be drastically reduced by phenytoin, which may put the patient at risk of graft rejection. In our case, the Drug Interaction Probability Scale6 suggested a probable association between initiation of phenytoin and an observed reduction in sirolimus levels. The patient needed a 4-fold increase in the dose of sirolimus to maintain drug levels within the target range. Metabolic induction occurs and dissipates slowly over a period of days to weeks, depending on the half-life of the inducer, the half-life of the CYP isozyme, the presence of hepatic disease, and the patient’s age.7 Phenytoin exhibits Michaelis–Menten kinetics, which makes it difficult to predict the duration of enzyme induction upon discontinuation, as clearance is concentration-dependent.8 To the authors’ knowledge, this is the first case in which a fall in GGT related to discontinuation of phenytoin was also correlated with a reduction in sirolimus dose. The GGT returned to baseline approximately 4 weeks after discontinuation of phenytoin.
The choice of an alternative antiepileptic drug will depend on several factors, including effectiveness of the drug for the particular seizure type, potential adverse effects, potential drug interactions, comorbid conditions (hepatic and renal disease), the patient’s age and lifestyle, the patient’s preferences, and cost. The patient described here had electroencephalographic and clinical evidence of partial and secondary generalized seizure. Broad-spectrum antiepileptic medications covering all seizure types include felbamate, lamotrigine, levetiracetam, topiramate, valproate, and zonisamide.9 There are no recognized differences in efficacy among the drugs used for treating newly diagnosed epilepsy.10 Of the available medications, levetiracetam has the most favourable adverse-effect profile.
The ideal course of action in a case such as ours would be to choose another anticonvulsant, so as to avoid the induction of hepatic enzymes caused by phenytoin, which in turn results in enhanced clearance of sirolimus. Levetiracetam is considered a valid alternative for patients who are receiving immunosuppressant therapy.11 It is not extensively metabolized, and 66% of the dose administered is excreted unchanged in the urine.12 In addition, about 24% of the dose administered is metabolized to an inactive metabolite that does not depend on hepatic CYP isozymes.12 Levetiracetam is not a high-affinity substrate for, nor is it an inhibitor of, the CYP isozymes, which suggests a low potential for drug interactions.12 Levetiracetam was effective in controlling the seizures of the patient described here.
1.
Zimmerman JJ. Exposure–response relationships and drug interactions of sirolimus.
AAPS J
2004;6(4):e28.
2.
Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation.
Drugs
2007;67(3):369–391.
3. Phenytoin. In: Lexi-Comp Online . Hudson (OH): Lexi-Comp, Inc; 2010 [cited 2010 Nov 30]. Available from: http://online.lexi.com. Subscription required to access content.
4.
Fridell JA, Jain AKB, Patel K, Virji M, Rao KN, Fung JJ, et al. Phenytoin decreases the blood concentrations of sirolimus in a liver transplant recipient: a case report.
Ther Drug Monit
2003;25(1):117–119.
5.
Hodges CB, Maxwell H, Beattie TJ, Murphy AV, Jindal RM. Use of rapamycin in a transplant patient who developed cyclosporine neurotoxicity.
Pediatr Nephrol
2001;16(10):777–778.
6.
Horn JR, Hansten PD, Chan LN. Proposal for a new tool to evaluate drug interaction cases.
Ann Pharmacother
2007;41(4):674–680.
7.
Formea CM, Evans CG, Karlix JL. Altered cytochrome P450 metabolism of calcineurin inhibitors: case report and review of the literature.
Pharmacotherapy
2005;25(7):1021–1029.
8. Winter TE. Phenytoin and fosphenytoin. In: Murphy JE, editor. Clinical pharmacokinetics . 4th ed. Bethesda (MD): American Society of Health-System Pharmacists; 2008. p. 247–264.
9. Schachter SC. Overview of the management of epilepsy in adults. In: UpToDate online [Internet database]. Version 19.1. Waltham (MA): UpToDate Inc; [updated 2011 Feb 2; cited 2011 April 13]. Available from: http.uptodateoneline.com. Subscription required to access content.
10.
Brodie MJ, Elder AT, Kwan P. Epilepsy later in life.
Lancet Neurol
2009;8(11):1019–1030.
11.
WinterSaiz Diaz RA, Sancho J, Serratosa J. Antiepileptic drug interactions.
Neurologist
2008;14(6 Suppl 1):S55–S65.
12. Keppra® (levetiracetam) [product monograph]. Oakville (ON): UCB Canada Inc; 2010 Sep 9.
* The patient gave informed consent for publication of this case report. ( Return to Text )
Canadian Journal of Hospital Pharmacy , VOLUME 64 , NUMBER 4 , July-August 2011