Use of Methadone for Prevention of Opioid Withdrawal in Critically Ill Children
Sonia A
Jeffries
,
Rumi
McGloin
,
Alexander F
Pitfield
,
Roxane R
Carr
ABSTRACT
Background
Opioids are commonly administered to critically ill children for analgesia and sedation, but many patients experience opioid withdrawal upon discontinuation. The authors’ institution developed a protocol for using methadone to prevent opioid withdrawal in children who have received morphine by continuous IV infusion for 5 days or longer in the pediatric intensive care unit (PICU).
Objectives
The primary objectives were to determine if opioids were tapered according to the protocol and to determine the conversion ratio for IV morphine to oral methadone that was used. Secondary objectives were to describe the methadone dosage used and the clinical outcomes, to evaluate adjustments to methadone dosing, and to report the incidence of adverse effects.
Methods
A retrospective analysis of charts was conducted for pediatric patients who had received morphine by continuous IV infusion for 5 days or longer followed by methadone in the PICU between May 2008 and August 2009. Validated scoring systems (the Withdrawal Assessment Tool and the State Behavioral Scale) were used to assess symptoms of withdrawal and degree of sedation, respectively.
Results
Forty-three patients were included in the study, with median age of 8 months (range 0.25–201 months). For 31 patients (72%), the protocol was not used, and there were no patients for whom the protocol was followed to completion. The median duration of weaning was 10 days (range 0–91 days). The conversion ratio for IV morphine to oral methadone was 1:0.78 for anticipated 5-day weaning and 1:0.98 for anticipated 10-day weaning. During the first 10 days of weaning, 18 patients (42%) experienced withdrawal symptoms. The methadone dose was increased for 11 (26%) of the 43 patients. Patients were sedated for a median of 1 day (range 0–9 days), were comfortable for a median of 6.5 days (range 1–64 days), and were agitated for a median of 2.5 days (range 0–23 days). Naloxone was required for 2 patients.
Conclusions
The institution’s methadone protocol was not followed consistently during the study period, and practices for transitioning from morphine by continuous IV infusion to methadone with tapering were also inconsistent. Further studies are needed to determine the optimal conversion ratio for morphine to methadone and the optimal tapering regimen to minimize withdrawal symptoms and adverse events.
KEYWORDS:
methadone
,
critical care
,
pediatrics
,
opioids
,
withdrawal
RÉSUMÉ
Contexte
Les opioïdes sont souvent administrés aux enfants gravement malades pour l’analgésie et la sédation, et beaucoup de ces patients subiront un syndrome de sevrage des opioïdes à l’arrêt du traitement. L’établissement des auteurs a développé un protocole d’utilisation de la méthadone pour prévenir le syndrome de sevrage des opioïdes chez les enfants qui ont reçu de la morphine par perfusion intraveineuse (i.v.) continue pendant cinq jours ou plus dans une unité de soins intensifs pédiatriques (USIP).
Objectifs
Les principaux objectifs étaient de déterminer si l’on diminuait graduellement les doses d’opioïdes selon le protocole et de déterminer le rapport de conversion de la morphine i.v. à la méthadone orale qui était utilisé. Les objectifs secondaires étaient de délimiter la posologie de la méthadone utilisée et les résultats cliniques, d’évaluer les ajustements posologiques de la méthadone et de constater l’incidence d’effets indésirables.
Méthodes
On a mené une analyse rétrospective des dossiers médicaux des enfants qui avaient reçu de la morphine en perfusion i.v. continue pendant au moins cinq jours, suivie de l’administration de méthadone, dans l’USIP entre mai 2008 et août 2009. On a utilisé des systèmes de notation (le
Withdrawal Assessment Tool
et le
State Behavioral Scale
) pour évaluer les symptômes de sevrage et le degré de sédation, respectivement.
Résultats
Au total, 43 patient ont été inclus dans l’étude; leur âge médian était de 8 mois (étendue : 0,25 à 201 mois). Le protocole n’a pas du tout été utilisé chez 31 (72 %) patients et n’a pas été suivi jusqu’à la fin chez aucun patient. La durée médiane du sevrage a été de 10 jours (étendue : 0 à 91 jours). Le rapport de conversion de la morphine i.v. à la méthadone orale était de 1:0,78 pour une période de sevrage prévue de cinq jours, et de 1:0,98 pour une période de sevrage prévue de 10 jours. Pendant les 10 premiers jours du sevrage, 18 (42 %) patients ont éprouvé des symptômes de sevrage. La dose de méthadone a été augmentée chez 11 (26 %) des 43 patients. Les patients ont souffert de sédation pendant une période médiane de 1 jour (étendue : 0 à 9 jours), étaient confortables pendant une période médiane de 6,5 jours (étendue : 1 à 64 jours) et étaient agités pendant une période médiane de 2,5 jours (étendue : 0 à 23 jours). Deux patients ont dû recevoir de la naloxone.
Conclusions
Le protocole d’utilisation de la méthadone à l’établissement des auteurs n’a pas été suivi systématiquement durant la période de l’étude. Les méthodes de transition de la morphine administrée par perfusion i.v. continue à la méthadone à doses dégressives étaient irrégulières. D’autres études sont nécessaires pour déterminer le rapport de conversion optimal de la morphine à la méthadone et le schéma dégressif optimal pour minimiser les symptômes de sevrage et les effets indésirables.
MOTS CLÉS:
méthadone
,
soins intensifs
,
pédiatrie
,
opioïdes
,
sevrage
[Traduction par l’éditeur]
INTRODUCTION
Opioids and benzodiazepines are commonly administered by continuous IV infusion in the pediatric intensive care unit (PICU) to manage symptoms of pain and anxiety, to provide sedation adequate to prevent accidental self-extubation and removal of catheters, and to reduce the morbidity associated with inadequate management of distress.1–3 Many critically ill children receive large doses of opioids and benzodiazepines over extended periods of time.
The probability of a patient experiencing withdrawal symptoms upon discontinuation of a medication increases with the cumulative dose and duration of therapy.2,4 Opioid withdrawal has been reported to occur after as few as 3 days of exposure by continuous IV infusion.5 Katz and others6 reported that children who received a continuous infusion of fentanyl in the PICU for more than 5 days had a greater than 50% chance of experiencing signs and symptoms of withdrawal. Receiving an infusion for 9 days or longer was 100% predictive of development of signs and symptoms of withdrawal.6
Opioid withdrawal syndrome may be difficult to diagnose, because issues such as inadequate analgesia or sedation, “ventilator-associated distress, and delirium can produce overlapping symptoms. Various scales for assessing withdrawal have been created in an attempt to distinguish opioid withdrawal symptoms from other effects.7,8 Commonly reported signs and symptoms of opioid withdrawal include agitation, irritability, fever, sweating, tremors, ataxia, dilatation of the pupils, visual and auditory hallucinations, frequent yawning, sneezing, high-pitched cry, hypertension, tachycardia, tachypnea, poor feeding, vomiting, and diarrhea.8,9 Various strategies have been used in attempts to prevent opioid withdrawal, such as pain assessment tools, adjunctive nonopioid analgesia, and non-pharmacologic methods to reduce the total amount of opioid medication used during periods of acute pain. When opioid analgesia is no longer required, other strategies include daily tapering of the dose (by 5% to 10% of the total daily dose) and use of long-acting medications such as methadone.2
The safe and effective use of methadone to wean children from opioids after long-term use in a critical care setting has been reported in several small studies.3,10–14 Methadone has several advantages over other oral opioids: it may be administered at longer intervals (every 6 to 12 h versus every 3 to 6 h for oral morphine); it is available in a liquid formulation; and it is well absorbed orally, with a bioavailability of about 70%–80%.15–17 In addition, methadone has a long half-life (15–60 h), and the half-life increases with repeated dosing.11,18,19 Because of the risk of accumulation and associated toxic effects, the dosing interval should be extended (e.g., from every 6 h to every 8 h) after the first 24 h of therapy.16 Despite the advantages of methadone, its pharmacokinetics and pharmacodynamics have not been well defined in children and are complicated by various disease states; by other medications; by the pharmacogenomic variability of the cytochrome P450 system; and by differences in body composition, metabolism, and clearance related to the child’s age.16,19 Another safety concern is the association of methadone with prolongation of the QTc interval, which led the US Food and Drug Administration to issue a warning in 2006.20
At the authors’ institution, morphine (administered by continuous IV infusion) is the standard opioid used for analgesia and sedation in the PICU. In May 2008, a protocol for using oral methadone to wean patients who have received morphine by continuous IV infusion for longer than 5 days was developed and implemented. According to the protocol, patients are to be weaned with tapering over 5 days (20% of original dose per day) or 10 days (10% of original dose per day), depending on the duration of continuous morphine infusion.
There is great variability in dosing of methadone in children, likely because of the paucity of data concerning use of this drug in this population. In particular, little information is available regarding the equivalence of IV morphine and oral methadone dosages in critically ill children, nor are the optimal weaning rate and frequency for critically ill children clear. This study aimed to describe the institution’s experience with methadone for weaning opioid-dependent children in the PICU. The primary objectives were to determine if tapering proceeded according to protocol for patients who received methadone in the PICU and to determine the conversion ratio for IV morphine to oral methadone that was used. The secondary objectives were to describe the methadone dosage used and the clinical outcomes, to evaluate the adjustments to methadone dosage made by physicians to control symptoms of withdrawal or toxicity, and to report the incidence of adverse effects.
METHODS
The study unit was the 22-bed PICU of the BC Children’s Hospital in Vancouver, British Columbia, which provides medical and surgical care to about 1700 critically ill infants and children annually.
The medical records of patients who received a continuous IV infusion of morphine followed by methadone in the PICU were evaluated retrospectively. Patients were identified through the institution’s pharmacy database. Patients 18 years of age or younger who received a continuous infusion of morphine for 5 days or longer and who received methadone for weaning from the opioid in the PICU between May 1, 2008, and August 31, 2009, were included. Patients were excluded if they had received methadone after continuous infusion of fentanyl or hydromorphone or if they had received methadone for neonatal abstinence syndrome.
The following information was collected for each patient: demographic characteristics, medical problems, concomitant medications, description of continuous infusion of morphine, conversion ratio for morphine to methadone, duration and description of methadone weaning schedule, assessment of sedation with the State Behavioral Scale,21 and assessment of withdrawal symptoms with the Withdrawal Assessment Tool (WAT-1).22 If no State Behavioral Scale score and/or WAT-1 score was recorded in the patient’s chart, a single designated investigator (S.A.J.) estimated the score (or scores) from the patient care flow sheet and medical record. The total amount of morphine that each patient received before weaning (total exposure) was calculated and divided by the number of days the patient was receiving morphine to determine the daily dose. Adverse drug reactions were assessed primarily by prolongation of the QTc interval and by the State Behavioral Scale score.
Descriptive statistics were used to report patient demographic characteristics, laboratory data, and outcome measures.
RESULTS
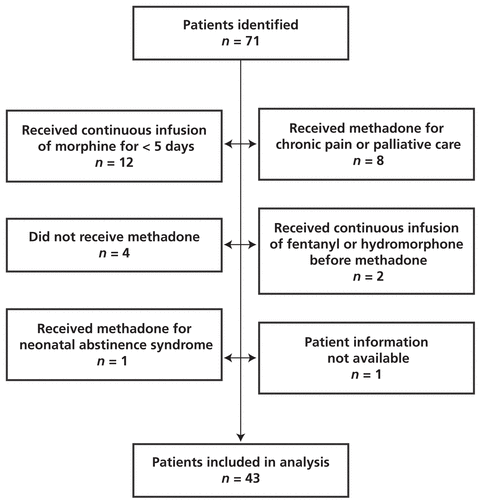
The database search identified a total of 71 patients as having received a continuous morphine infusion followed by methadone in the PICU between May 1, 2008, and August 31, 2009. Of these, 43 patients met the inclusion criteria (Figure 1).
| |
|
|
|
Figure 1
Identification of pediatric patients meeting inclusion criteria for study of methadone following continuous IV infusion of morphine. The initial group of potentially eligible patients was identified by searching the pharmacy’s patient database for patients who received 5 days of opioid therapy followed by methadone; subsequent examination of patients’ medical records revealed that some of the patients had not actually received the drugs recorded in the computer system.
|
Patients ranged in age from 1 week to 17 years, and about two-thirds were boys (Table 1). The most common reasons for admission to the PICU were respiratory failure (18 patients [42%]), postoperative congenital heart disease (14 [33%]), brain injury (4 [9%]), and septic shock (3 [7%]). Fifteen (35%) of the patients also had one or more additional clinically significant medical problems, including renal failure in 8 patients (19%), requirement for extracorporeal life support in 6 patients (14%), heart arrhythmia in 4 patients (9%), in utero exposure to opioids in 1 patient (2%), and heart transplant in 1 patient (2%). None of the patients had evidence of liver dysfunction. Twenty patients (47%) received medications with documented interactions with methadone (Table 2).23
Table 1
Demographic Characteristics of 43 Patients
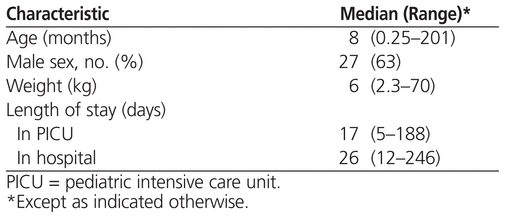
Table 2
Medications Known to Interact with Methadone23 That Were Administered to Patients in the Study

The median daily dosage of opioid in morphine equivalents was 0.79 mg/kg among patients eligible for 5-day weaning and 0.71 mg/kg among patients eligible for 10-day weaning (Table 3). The median duration of opioid use before initiation of methadone for both groups combined was 9 days (range 5–24 days). According to the protocol, the dosage of methadone on day 0 is to be based on the amount of morphine received in the 24 h preceding conversion. In this study, the median amount of morphine received in the 24 h preceding conversion was 0.75 mg/kg (range 0–2.82 mg/kg). For one patient, the administration of morphine was stopped after more than 5 days of continuous IV infusion, with the methadone weaning protocol being started more than 24 h later, when the patient started to experience withdrawal symptoms. As such, this patient had received no opioids within the preceding 24 h. Among all patients, bolus doses of morphine accounted for a median of 7.2% (range 0%–66%) of the 24-h dose preceding initiation of methadone.
Table 3
Total Opioid Use and Methadone Conversion Ratio
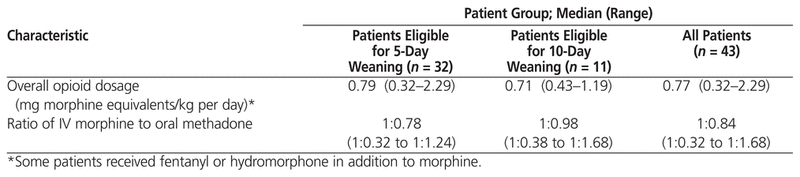
For 31 patients (72%), administration of methadone did not follow the protocol, primarily because the conversion ratio used was different from the protocol-specified ratio of 1:1 (i.e., 1 mg morphine to 1 mg methadone) for 29 patients (67%). For 7 patients (16%), the initial dosing interval was every 8 h (rather than every 6 h). Even among the 12 patients for whom the protocol was initiated, it was not followed to completion. The ratio of IV morphine to oral methadone was close to the recommended 1:1 ratio for the 11 patients (26%) eligible for 10-day weaning (ratio 1:0.98), but less methadone than recommended was used for the 32 patients (74%) eligible for 5-day weaning (ratio 1:0.78) (Table 3). Among all patients, the median duration of weaning was 10 days (range 0–91 days). Those eligible for 5-day weaning were tapered over a median of 9 days (range 0–48 days); those eligible for 10-day weaning were tapered over a median of 15 days (range 7–91 days).
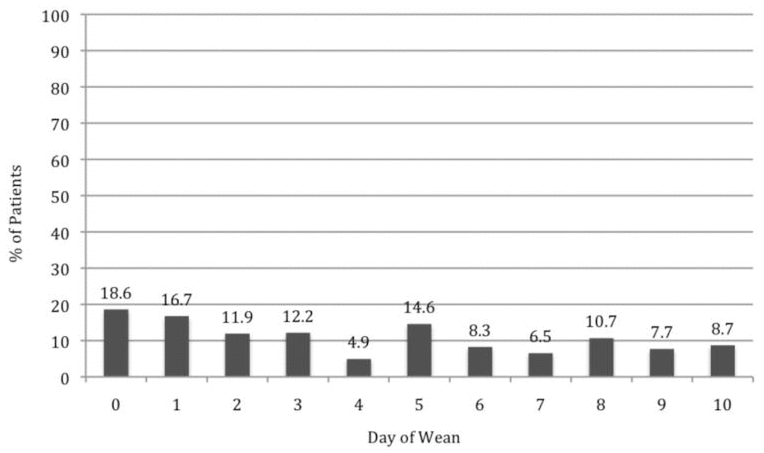
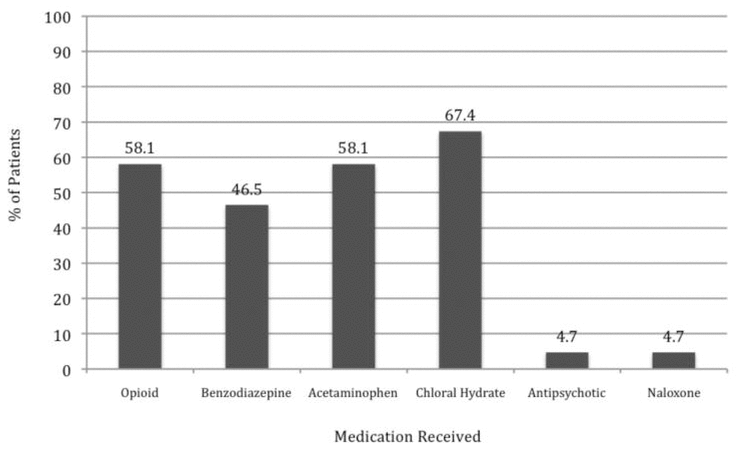
Patients with a WAT-1 score of 3 or above are considered to be experiencing withdrawal symptoms.22 In this study, we determined that 18 patients (42%) had a WAT-1 score of 3 or greater at some point during the first 10 days of methadone weaning, as described in further detail in Figure 2. During the weaning period, an increase in methadone dose was required for 11 patients (26%), one or more doses of methadone had to be held for 7 patients (16%), and a temporary switch to IV morphine was required for 4 patients (9%). Numerous adjunctive medications were given throughout the methadone weaning period (Figure 3).
| |
|
|
|
Figure 2
Percentage of patients with Withdrawal Assessment Tool score of 3 or above in first 10 days of weaning. The number of patients for calculating these percentages (i.e., the denominator) ranged from 23 to 43.
|
| |
|
|
|
Figure 3
Use of adjunctive medications during weaning. Percentages were calculated on the basis of all 43 patients in the study sample.
|
One patient experienced prolongation of the QTc interval, but monitoring by electrocardiography (ECG) was documented for only 5 patients. According to the State Behavioral Scale, patients were sedated (score ≤ −1) for a median of 1 day (range 0–9 days), comfortable (score = 0) for a median of 6.5 days (range 1–64 days), and agitated (score ≥ 1) for a median of 2.5 days (range 0–23 days). For 2 patients (5%), administration of naloxone was required.
DISCUSSION
This study revealed that, during the defined study period, the methadone weaning protocol developed by the study institution was not being followed in the PICU. The points of non-compliance included initial conversion from IV morphine to oral methadone and rate of subsequent decreases in dosage. The protocol specifies a 1:1 conversion ratio for IV morphine to oral methadone, regardless of the duration of morphine therapy preceding conversion. Specification of this ratio in the protocol was based on the conversion ratio for oral morphine to IV morphine (3:1),24 the conversion ratio for oral morphine to oral methadone (3:1),25 and clinicians’ prior experience with these medications.
The conversion to methadone from other opioids is complicated by the complex pharmacokinetic profile and variable equianalgesic dose ratio for this drug.24–26 Numerous approaches to guide conversion from another opioid to oral methadone have been described in the literature; however, most of the literature involves conversion from long-term IV fentanyl infusion, and there is no consensus regarding the conversion factor. In addition, the documented methods of conversion are inconsistent, for example, starting all patients (regardless of prior opioid exposure) with the same initial oral methadone dose of 0.1 mg/kg per dose every 12 h7,18 or conversion ratio for IV fentanyl to oral methadone of 1 mg to 3 mg.15,18,25 The literature is also variable in terms of dosing interval for methadone, dosing decrements (e.g., daily decreases or 20% decrease on a weekly basis), and duration of weaning.3,9–14,26–28
In this study, for patients eligible for 5-day weaning (i.e., had received morphine by continuous IV infusion for 5 to 10 days), the median ratio of morphine to oral methadone was 1 mg to 0.78 mg, whereas for those eligible for 10-day weaning (i.e., had received morphine by continuous IV infusion for more than 10 days), the median ratio was 1 mg to 0.98 mg. Although the reasons for not using the recommended 1 to 1 ratio are unclear, we speculated that bolus doses of morphine received in the 24 h before conversion were not accounted for by clinicians doing the conversion.
This study revealed that bolus doses of morphine accounted for up to 66% of the total morphine dose in the 24 h preceding initiation of methadone. The weaning protocol states that bolus doses should be included in the calculation of morphine exposure. The clinical significance of not including bolus doses when converting to methadone could not be specifically determined from this study but may partially explain the rate of withdrawal symptoms seen in the first 24 h (day 0, before weaning was initiated) (Figure 2).
The protocol attempts to optimize the pharmacokinetic and pharmacodynamic characteristics of methadone by specifying that the dosing interval be adjusted after the first 24 h of therapy (i.e., the equivalent daily methadone dose is initially divided and given every 6 h, with the interval being extended, to every 8 h, 24 h later). An interval of every 6 h is needed initially to ensure accumulation of methadone in the tissues; otherwise, methadone’s duration of effect is unlikely to span the entire 8 h of the longer dosing interval. In some instances, the pharmacokinetic principles of methadone were not taken into account; more specifically, 16% of the children were started on methadone at a dosing interval of every 8 h.
During the first 10 days of the methadone weaning period, 42% of the patients in this study experienced withdrawal symptoms. As described above, alteration of the initial dosing ratio and interval from what is recommended in the protocol may have played a role in the occurrence of these symptoms. In addition to the issues described above regarding inclusion of bolus doses of morphine in the 24-h morphine calculation, it is possible that the 1:1 ratio suggested by the protocol is less than optimal. Either or both of these factors may account for the large number of patients experiencing withdrawal symptoms on day 0 of the weaning period, even though the amount of methadone given on day 0 should have been equivalent to the amount of morphine given over the previous 24 h. The ideal conversion ratio of IV morphine to oral methadone for critically ill children is as yet unknown, and further prospective study is needed to determine optimal conversion and dosage weaning strategies.
In addition, some patients may have been converted to methadone therapy while they still required management of acute pain and sedation. Premature conversion might account for some of the cases in which the methadone dose was increased during the weaning period. Switching to methadone while acute pain is an issue might also have been a factor in the prolonged duration of methadone weaning (10 days, despite the fact that the majority of the children were eligible for 5-day weaning).
The current protocol does not prescribe assessments of the patient’s status (including assessment of withdrawal symptoms), nor does it provide standardized adjustments if withdrawal symptoms or toxic effects are noted. Standard PICU procedures require that nursing staff monitor sedation and analgesia every 4 h. Although the State Behavioral Scale and WAT-1 assessments were available to clinicians during the period studied, their use was inconsistent. Documentation was scarce, and other scoring systems were also being used to monitor patients in this study. If there were signs of too much or inadequate sedation or analgesia or signs of withdrawal, the physician altered therapy according to need. However, the reasons for any therapeutic alterations made were not documented in any patient’s chart, nor was an objective tool consistently used to assess withdrawal symptoms. Assessment of sedation with the State Behavioral Scale was not correlated with methadone dosing and was not consistently recorded as having been assessed. Although an increase in methadone dose was required for some patients, and the methadone dose had to be held for others, no patient required a methadone dose to be held after an increase in dose.
A warning from the US Food and Drug Administration in 2006 and a subsequent expert panel recommendation in 2009 addressed concerns about prolongation of the QTc interval and increased risk of torsade de pointes with the use of methadone.20,29,30 Since then, the decision to perform ECG before and during treatment (for screening purposes) has remained controversial. During the study period, routine ECG monitoring was not standard practice for patients receiving methadone at the authors’ institution. Only 5 patients were documented to have undergone ECG while receiving methadone. The single patient who had documented prolongation of the QTc interval did not experience any clinically detrimental effects.
Patients experienced more days of agitation (median 2.5 days) than oversedation (median 1 day) during the weaning period; however, 2 patients required the use of naloxone. One patient received naloxone on day 2 of the weaning period, had a good response, and was able to complete the methadone weaning period with lower doses. The other patient received naloxone on day 1 of the weaning period, had a good response, and received no further doses of methadone. The outcomes for these patients highlight the highly variable pharmacokinetics and pharmacodynamics of methadone.
This study had some limitations inherent to its retrospective design. The protocol had been in place for a relatively short period at the time of the study, and only a small number of cases were eligible for inclusion in the review. Some patient information was incomplete or unavailable; therefore, the clinical rationale for changes made during the methadone weaning period may not have been fully understood. A single investigator used information available in the patient’s chart to estimate missing values for the State Behavioral Scale and the WAT-1, both of which are validated scoring systems. Incomplete data were unlikely to have affected the WAT-1 scores, as the presence or absence of fever, loose stools, and vomiting (used in the WAT-1 score) were always documented in the patient’s chart. These key symptoms alone allowed for the diagnosis of opioid withdrawal, since a WAT-1 score of 3 or greater signified withdrawal. The methadone protocol was not followed to completion for any patient. As such, the efficacy and safety of the protocol could not be evaluated.
This study showed that the practice of weaning methadone at the study institution was inconsistent during the study period.
The addition of regular patient assessments and the implementation of standardized adjustments when symptoms of withdrawal or toxicity are noted might improve the usability of the protocol and increase the consistency of methadone tapering. Further studies are needed to determine the optimal conversion ratio of IV morphine to oral methadone, the ideal duration of the methadone weaning period, and the weaning rate and frequency that would minimize withdrawal symptoms and adverse effects.
References
1
Franck LS, Naughton I, Winter I. Opioid and benzodiazepine withdrawal symptoms in paediatric intensive care patients.
Intensive Crit Care Nurs
2004;20(6):344–351.


2
Playfor SD. Analgesia and sedation in critically ill children.
Contin Educ Anaesth Crit Care Pain
2008;8(3):90–94.

3
Berens RJ, Meyer MT, Mikhailov TA, Colpaert KD, Czarnecki ML, Ghanayem NS, et al. A prospective evaluation of opioid weaning in opioid-dependent pediatric critical care patients.
Anesth Analg
2006;102(4):1045–1050.


4
Redmond DE Jr, Krystal JH. Multiple mechanisms of withdrawal from opioid drugs.
Annu Rev Neurosci
1984;7:443–478.


5
Ducharme C, Carnevale FA, Clermont MS, Shea S. A prospective study of adverse reactions to the weaning of opioids and benzodiazepines among critically ill children.
Intensive Crit Care Nurs
2005;21(3):179–186.

6
Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion.
Crit Care Med
1994;22(5):763–767.


7
Anand KJS, Arnold JH. Opioid tolerance and dependence in infants and children.
Crit Care Med
1994;22(2):334–342.


8
Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M. Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: a first evaluation.
Crit Care Med
2008; 36(8):2427–2432.


9
Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit.
Crit Care Med
2000;28(6):2122–2132.


10
Tobias JD, Deshpande JK, Gregory DF. Outpatient therapy of iatrogenic drug dependency following prolonged sedation in the pediatric intensive care unit.
Intensive Care Med
1994;20(7):504–507.


11
Lugo RA, MacLaren R, Cash J, Pribble CG, Vernon DD. Enteral methadone to expedite fentanyl discontinuation and prevent opioid abstinence syndrome in the PICU.
Pharmacotherapy
2001;21(12):1566–1573.

12
Siddappa R, Fletcher JE, Heard AMB, Kielma D, Cimino M, Heard CMB. Methadone dosage for prevention of opioid withdrawal in children.
Paediatr Anaesth
2003;13(9):805–810.


13
Meyer MT, Berens RJ. Efficacy of an enteral 10-day methadone wean to prevent opioid withdrawal in fentanyl-tolerant pediatric intensive care unit patients.
Pediatr Crit Care
Med 2001;2(4):329–233.

14
Robertson RC, Darsey E, Fortenberry J, Pettignano R, Hartley G. Evaluation of an opiate-weaning protocol using methadone in pediatric intensive care unit patients.
Pediatr Crit Care Med
2000;1(2):119–123.

15
Buck ML. Managing iatrogenic opioid dependence with methadone.
Pediatr Pharmacother
2000 July;6(7).
16
Lugo RA, Satterfield KL, Kern SE. Pharmacokinetics of methadone
. J Pain Palliat Care Pharmacother
2005;19(4):13–24.

17
Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence.
Clin Pharmacokinet
2002;41(14):1153–1193.


18
Berdine HJ, Nesbit SA. Equianalgesic dosing of opioids
. J Pain Palliat Care Pharmacother
2006;20(4):79–84.


19
Yang F, Tong X, McCarver DG, Hines RN, Beard DA. Population-based analysis of methadone distribution and metabolism using an age-dependent physiologically based pharmacokinetic model.
J Pharmacokinet Pharmacodyn
2006;33(4):485–518.


20
Methadone hydrochloride (marketed as Dolophine) information: death, narcotic overdose, and serious cardiac arrhythmias [FDA Alert]. Rockville (MD): US Food and Drug Administration; 2006 Nov [cited 2011 Jan 5]. Available from: www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm142827.htm


21
Curley MAQ, Harris SK, Fraser KA, Johnson RA, Arnold JH. State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation.
Pediatr Crit Care Med
2006;7(2):107–114.



22
Franck LS, Harris SK, Soetenga DJ, Amling JK, Curley MAQ. The Withdrawal Assessment Tool–1 (WAT–1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients.
Pediatr Crit Care Med
2008;9(6):573–580.


23
Methadone. In: Lexi-InteractTM [database]. Hudson (OH): Lexi-Comp Inc.; [no date; cited 2011 Jan 5]. Available from: www.lexi.com/tours/online.jsp?id=lco16. Subscription required to access content.
24
Gammaitoni AR, Fine P, Alvarez N, McPherson ML, Bergmark S. Clinical application of opioid equianalgesic data.
Clin J Pain
2003;19(5):286–297.


25
Zimmermann C, Seccareccia D, Booth CM, Cottrell W. Rotation to methadone after opioid dose escalation: How should individualization of dosing occur?
J Pain Palliat Care Pharmacother
2005;19(2):25–31.

26
Ripamonti C, Groff L, Brunelli C, Polastri D, Stavrakis A, De Conno F. Switching from morphine to oral methadone in treating cancer pain: What is the equianalgesic dose ratio?
J Clin Oncol
1998;16(10):3216–3221.

27
Linderbeck LR. Update of the clinical issues regarding methadone (Dolophine) therapy in pain management.
AACN Adv Crit Care
2008;19(3):253–257.


28
Tobias JD, Schleien CL, Haun SE. Methadone as treatment for iatrogenic narcotic dependency in pediatric intensive care unit patients.
Crit Care Med
1990;18(11):1292–1293.


29
Dolophine® hydrochloride CII (methadone hydrochloride tablets, USP) 5 mg, 10 mg Rx only [package insert]. Columbus (OH): Roxane Laboratories Inc; 2006 [cited 2011 Jan 5]. Available from: www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM142842.pdf


30
Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MCP. QTc interval screening in methadone treatment.
Ann Intern Med
2009;150(6):387–395.

Sonia A Jeffries, BScPharm, is with the Children’s & Women’s Health Centre of British Columbia, Vancouver, British Columbia
Rumi McGloin, PharmD, is with the Children’s & Women’s Health Centre of British Columbia, Vancouver, British Columbia
Alexander F Pitfield, MD, is with the Children’s & Women’s Health Centre of British Columbia, Vancouver, British Columbia
Roxane R Carr, PharmD, BCPS, FCSHP, is with the Children’s & Women’s Health Centre of British Columbia and the Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, British Columbia
Address correspondence to: Dr Roxane R Carr, Department of Pharmacy, Room 0B7, Children’s & Women’s Health Centre of British Columbia, 4500 Oak Street, Vancouver BC V6H 3N1, e-mail:
rcarr@cw.bc.ca
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
65
, NUMBER
1
, January-February 2012








