Denise Reniers , Irina Rajakumar , Suzanne Ratko , Paul Atkison
Published data assessing the efficacy and toxicity of omega-3 fatty acid emulsion in children are limited.1–3 Until recently, children requiring long-term parenteral nutrition who experienced parenteral nutrition–associated liver disease (PNALD) were likely to die from infection or liver disease before receiving a small bowel and/or liver transplant.4 With the introduction of parenteral fish oil (10% long-chain omega-3 fatty acid emulsion; Omegaven, Fresenius Kabi, Bad Homburg, Germany), originally indicated for use in combination with a soybean oil–based lipid emulsion (Intralipid), we have been able to reverse this life-threatening condition in several infants without the need for transplantation. We present our experience with parenteral administration of fish oil in 8 infants with PNALD. Verbal informed consent for publication was obtained from the patients’ guardians. No ethics approval was required by our institution.
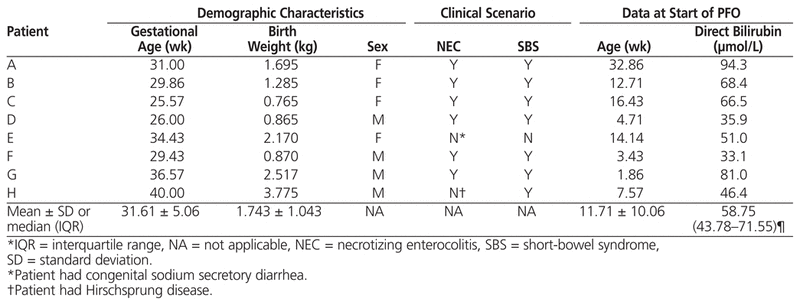
This case series describes the first 8 consecutive infants at the authors’ institution for whom therapy with parenteral fish oil was started (August 2006 to September 2008) after development of hepatic cholestasis associated with parenteral nutrition. Six of the patients had necrotizing enterocolitis and short-bowel syndrome, one patient had very long segment Hirschsprung disease with short-bowel syndrome, and one patient had congenital sodium secretory diarrhea and was dependent on parenteral nutrition. Demographic characteristics and direct bilirubin values at the start of parenteral fish oil therapy are detailed in Table 1.
Table 1
Baseline Characteristics of 8 Infants who Received Parenteral Fish Oil (PFO)

For most patients, parenteral fish oil was started when direct bilirubin was greater than 35 μmol/L. In one case, the parenteral fish oil was started in the neonatal intensive care unit at another institution at a slightly lower bilirubin level (33 μmol/L). In all cases, the parents gave verbal consent before parenteral fish oil emulsion was started. The emulsion was initiated at 0.5 g/kg and was increased to 1 g/kg after 2 days.5 The emulsion was prepared in 60-mL syringes to allow delivery via a syringe pump, with an expiry time of 24 h at room temperature. The infusion was interrupted as required for administration of other medications. The parenteral fish oil was administered for a period ranging from 4.6 to 8.4 months. All patients had previously received up to 3 g/kg per day of IV Intralipid, which was discontinued when the parenteral fish oil was started. Six of the 8 infants were receiving part of their caloric intake in the form of enteral feeding, which may in part stimulate bile flow and help to reduce bilirubin.
After the introduction of IV parenteral fish oil therapy, liver enzyme values (specifically aspartate aminotransferase) were normalized at a median of 68 days (interquartile range [IQR] 57–111 days). Total bilirubin normalized (range 3.4–17.1 μmol/L) at a median of 99 days (IQR 75–113 days) and direct bilirubin at 130 days (IQR 115–135 days). However, direct bilirubin values were approaching normal (< 5.1 μmol/L) by day 90 of therapy in most infants (Figure 1). For one of the patients (patient F), normalization took longer, presumably because of an underlying liver disease that was still undiagnosed at the time of writing. Liver biopsy suggested biliary atresia, which has since been ruled out.
|
|
||
|
Figure 1 Bilirubin values for individual patients in the case series. |
||
The parenteral fish oil therapy was well tolerated and was not associated with any infusion-related adverse events or delayed growth. There were no clinical symptoms of essential fatty acid deficiency, such as dermatitis. However, biochemical testing for such deficiency was not performed because of a lack of availability of such testing in Canada. Patient F had some rectal bleeding associated with elevation of the international normalized ratio (INR), but there was no change in hemoglobin, and transfusion was not required.
PNALD occurs in 40% to 60% of infants who require parenteral nutrition for 1 to 3 months, with the incidence increasing to 85% among those who receive parenteral nutrition for more than 100 days.1,6 Some evidence suggests that infants with short-bowel syndrome and direct bilirubin levels remaining above 3 mg/dL (51.3 μmol/L) for more than 3 months are at increased risk of death from liver failure.5 Furthermore, direct bilirubin above 4 mg/dL (68.4 μmol/L) for longer than 6 months was correlated with a mortality rate of 78%.7 Most patients in the current case series had direct bilirubin values approaching normal by day 90 of therapy with parenteral fish oil (Figure 1).
Although parenteral fish oil is generally available in Europe, it is available in Canada and the United States only with approval from the pertinent federal regulatory body. This type of therapy should not be administered to patients with allergy to fish or egg protein and should be used with caution in patients requiring anticoagulant therapy, as the parenteral fish oil may prolong bleeding time and inhibit platelet aggregation.8 Of note in the current case series, patient F had abnormal values for INR and partial thromboplastin time (PTT) during therapy with parenteral fish oil because of bleeding problems during a period when results for bilirubin and liver function tests had normalized. The INR normalized with parenteral administration of vitamin K. At the study institution, INR and PTT values are not routinely measured for patients with otherwise normal liver enzymes and function who are receiving parenteral nutrition; as such, we do not have values for other patients to which comparisons could be made.
All of the infants in this case series experienced bacterial infections with enteric organisms, which is a common complication of long-term parenteral nutrition through a central venous catheter.7 One of the patients (patient A) experienced neutropenic episodes while in hospital (neutrophil count 0.5 × 109/L to 1.0 × 109/L), but these episodes did not directly correlate with the onset of bacterial infection or initiation of parenteral fish oil therapy and persisted after the therapy was discontinued. Three of the patients (patients A, B, and G) required oral selenium supplementation. Parenteral nutrition with trace elements was started for all patients; however, copper and manganese were excluded to limit potential worsening of PNALD and accumulation of these trace minerals.1,7 Children receiving long-term parenteral nutrition should undergo serum micronutrient monitoring to ensure adequate intake and to prevent accumulation.
The cause of PNALD is not well understood. Proposed mechanisms include the use of soybean and safflower oil–based fat emulsions rich in omega-6 fatty acids, which have pro-inflammatory properties potentially leading to liver injury. Phytosterols found in soybean lipid emulsions are postulated to reduce bile flow, leading to formation of biliary sludge. In one study, children with severe parenteral nutrition–associated cholestasis had high phytosterol levels,7 but no direct correlation has been demonstrated between phytosterols and development of cholestasis in patients receiving lipid emulsions.4 Parenteral fish oil contains mostly omega-3 fatty acids, which have anti-inflammatory properties and lack phytosterols, and it is the presence of this type of fatty acid that may play a role in improving bile flow and improving hepatic cholestasis. In a previous study, the use of parenteral fish oil in a small subset of patients resulted in a shorter time to reversal of cholestasis relative to patients who received soybean oil emulsion, with no difference in essential fatty acid levels or infection rates.3
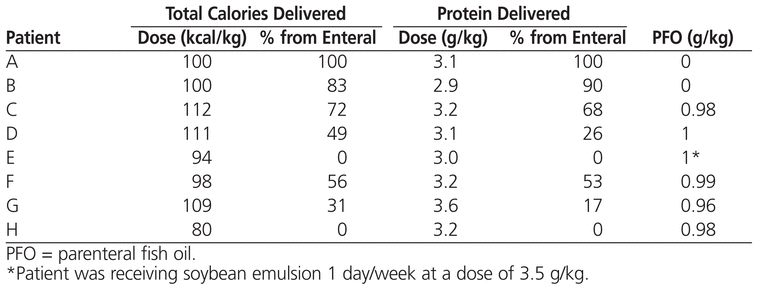
In the study reported here, the use of parenteral fish oil reversed parenteral nutrition–induced cholestasis in all 8 patients over a median period of 18.6 weeks. The median time to normalization in previously published reports has ranged from 8 weeks to 8 months.2,3,5 In 2 patients, serum bilirubin levels normalized without enteral nutrition (patients E and H; see Table 2), which correlates with previously reported results.3 Reversal of cholestasis may also be achieved by limiting or temporarily withholding soybean oil emulsion.9,10
Table 2
Nutrition When Bilirubin Values Normalized
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are derivatives of linolenic acid. Linolenic acid (along with linoleic acid) is an essential fatty acid necessary for brain development. EPA and DHA make up greater than 50% of the fatty acid content of parenteral fish oil.11 There has been concern that utilization of parenteral fish oil may lead to essential fatty acid deficiency. In a previously published case series, one patient exhibited biochemical evidence of essential fatty acid deficiency at 4 weeks, despite a lack of clinical evidence.3 The patient was not receiving parenteral fish oil therapy at the time of these abnormal results. Parenteral fish oil was reintroduced, and the biochemical markers returned to normal. Other reports of patients with essential fatty acid deficiency while receiving parenteral fish oil alone (1 g/kg) have been published.12–14 In view of this potential problem, patient E was given Intralipid (3.5 g/kg) 1 day/week, as she was unable to tolerate even small amounts of enteral feeds and was therefore fed solely by parenteral nutrition. At the time of writing, the long-term outcomes for children maintained on parenteral fish oil as their sole source of fat is generally unknown.
To date, only a few case reports of success with parenteral fish oil for hepatic cholestasis have been published.2,3,5 Further studies are needed to determine if initiation of treatment with this form of therapy, alone or in combination with soybean oil emulsion, in cases with anticipated long-term need for parenteral nutrition, will prevent hepatic cholestasis. In addition, larger studies in the pediatric population are needed to further evaluate the safety and efficacy of parenteral fish oil for the prevention and reversal of hepatic dysfunction secondary to parenteral nutrition.3 A prospective, randomized trial is currently under way to evaluate and compare the efficacy of fish oil–based emulsion and conventional soybean oil emulsion in the prevention of cholestasis.15 These data will facilitate decision-making regarding the appropriate time to initiate therapy with parenteral fish oil.
1
Heine RG, Bines JE. New approaches to parenteral nutrition in infants and children.
J Paediatr Child Health
2002;38(5):433–437.
2
Ekema G, Falchetti D, Boroni G, Tanca AR, Altana C, Righetti L, et al. Reversal of severe parenteral nutrition-associated liver disease in an infant with short bowel syndrome using parenteral fish oil (omega-3 fatty acids).
J Pediatr Surg
2008;43(6):1191–1195.
3
Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition–associated liver disease.
Pediatrics
2008;121(3):e678–e686.
4
Atkison PR, Ross BC, Williams S, Howard J, Sommerauer J, Quan D, et al. Long-term results of pediatric liver transplantation in a combined pediatric and adult transplant program.
CMAJ
2002;166(13):1663–1671.

5
Gura KM, Duggan CP, Collier SB, Jennings RW, Folkman J, Bistrian BR, et al. Reversal of parenteral nutrition–associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management.
Pediatrics
2006;118(1):e197–e201.
6
Christensen RD, Henry E, Wiedmeier SE, Burnett J, Lambert DK. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition–associated liver disease.
J Perinatol
2007;27(5):284–290.
7
Btaiche IF, Khalidi N. Parenteral nutrition–associated liver complications in children.
Pharmacotherapy
2002;22(2):188–211.
8
Omegaven® product information. Bad Homburg (Germany): Fresenius Kabi; 1998 (revised 2009).

9
Rollins MD, Scaife ER, Jackson WD, Meyers RL, Mulroy CW, Book LS. Elimination of soybean lipid emulsion in parenteral nutrition and supplementation with enteral fish oil improve cholestasis in infants with short bowel syndrome.
Nutr Clin Pract
2010;25(2):199–204. Erratum in:
Nutr Clin Pract
2010;25(3):315.
10
Colomb V, Jobert-Giraud A, Lacaille F, Goulet O, Fournet JC, Ricour C. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children.
JPEN J Parenter Enteral Nutr
2000;24(6):345–350.
11 Krebs NF, Primak LE, Haemer M. Normal childhood nutrition. In: Hay W, Levin M, Sondheimer J, Deterding R, editors. Current diagnosis and treatment in pediatrics. New York (NY): McGraw-Hill Companies, Inc; 2011. p. 273–299.
12 Groleau V, Thibault M, Marchand V. Use of fish oil emulsion in parenteral nutrition: a review of 31 cases [abstract]. 6th International Pediatric Intestinal Failure and Rehabilitation Symposium; 2010 Sep 9–11; Chicago (IL). p. 127.
13 Sigalet D, Boctor D, Lam V, Brindle M, Rosemary J, Sharla S, et al. Aggressive prevention of liver disease improves intestinal rehabilitation [abstract]. 6th International Pediatric Intestinal Failure and Rehabilitation Symposium; 2010 Sep 9–11; Chicago (IL). p. 130.
14 Bibas M, Pineault M, Bouthillier L, Marchand V. Early use of omegaven in short bowel patients on parenteral nutrition: transient cholestasis, liver fibrosis and essential fatty acid deficiency [abstract]. 6th International Pediatric Intestinal Failure and Rehabilitation Symposium; 2010 Sep 9–11; Chicago (IL). p. 141.
15
de Meijer VE, Gura KM, Le HD, Meisel JA, Puder M. Fish oil-based lipid emulsions prevent and reverse parenteral nutrition–associated liver disease: the Boston experience.
JPEN J Parenter Enteral Nutr
2009;33(5):541–547.
Canadian Journal of Hospital Pharmacy , VOLUME 65 , NUMBER 1 , January-February 2012