Jennifer G Kendrick , Kelly Ma , Pia DeZorzi , Don HamiltonABSTRACT
Background:
At the time of this study, the authors’ pediatric tertiary care hospital had no policy to guide actions when a child vomited after ingesting oral medication, and limited information was available in the literature.
Objectives:
To characterize this clinical problem at the study hospital, to identify current practices related to redosing of medications at the study hospital, and to collect guidelines and opinions of health care professionals at other pediatric hospitals on this topic.
Methods:
Two online surveys were conducted, each over a 1-month period in late 2010, to identify current practices and opinions of pediatric health care professionals about redosing of medications after vomiting. E-mail distribution lists and health care forums were used to recruit participants.
Results:
Of the 76 responses from the study hospital, 65 were suitable for analysis. Many respondents reported encountering vomiting after administration of oral medications on a weekly (25 [38%]) or monthly (24 [37%]) basis. Most of the respondents reported that they would follow a general rule to redose if vomiting occurred within 30 min (39 [60%]) or 15 min (21 [32%]) after initial ingestion. When respondents were asked to rate the importance of 8 factors potentially affecting the decision to redose, more than half indicated that time after dose ingestion (59 [91%]), medication type (45 [69%]), patient status (39 [60%]), and visibility of medication in the vomitus (36 [55%]) were very important. Of the 53 respondents to the survey of health care professionals at other institutions, 16 (30%) indicated that their pediatric hospital or ward had a guideline on redosing in cases of vomiting after administration of oral medications. Most respondents (12/13 [92%]) stated that the guideline took into account the interval between initial ingestion and vomiting.
Conclusions:
The problem of vomiting after administration of an oral medication was prevalent at the study hospital, and guidelines were scarce at other pediatric institutions. Health care professionals at the study hospital and other institutions listed the time between ingestion and vomiting as the most important factor in the decision to redose the medication.
KEYWORDS: vomiting , pediatric , oral medication , survey
RÉSUMÉ
Contexte :
Au moment de cette étude, l’hôpital de soins tertiaires pour enfants où les auteurs pratiquent n’avait aucune politique guidant les mesures à prendre chez un enfant qui vomissait après l’ingestion de médicaments par voie orale, et on trouvait peu d’information sur ce sujet dans la littérature.
Objectifs :
Caractériser ce problème clinique à l’hôpital en question, déterminer les pratiques actuelles de réadministration des médicaments et consigner les lignes directrices et les opinions pertinentes des professionnels de la santé d’autres hôpitaux pour enfants.
Méthodes :
Deux sondages en ligne ont été effectués, chacun sur une période d’un mois à la fin de 2010, afin de définir les pratiques et les opinions actuelles des professionnels des soins de santé pédiatriques concernant la réadministration des médicaments après les vomissements. Des listes de diffusion par courriel et des forums de soins de santé ont servi à recruter les participants.
Résultats :
Des 76 réponses reçues à cet hôpital, 65 ont été retenues aux fins d’analyse. De nombreux répondants ont déclaré rencontrer des cas de vomissements après l’administration de médicaments par voie orale sur une base hebdomadaire (25 [38 %]) ou mensuelle (24 [37 %]). La plupart des répondants ont déclaré observer une règle générale voulant qu’on réadministre le médicament si l’enfant vomissait dans les 30 minutes (39 [60 %]) ou les 15 minutes (21 [32 %]) suivant l’administration initiale du médicament. En réponse à la question sur l’importance relative de 8 facteurs ayant une incidence possible sur la décision de réadministrer le médicament, plus de la moitié des répondants ont indiqué que le temps écoulé après l’ingestion du médicament (59 [91 %]), le type de médicament (45 [69 %]), l’état du patient (39 [60 %]) et la visibilité du médicament dans les vomissements (36 [55 %]) étaient très importants. Des 53 répondants du sondage auprès des professionnels de la santé d’autres établissements, 16 (30 %) ont indiqué que leur hôpital pour enfants ou le service de soins aux enfants possédait des lignes directrices sur la conduite à tenir en cas de vomissements après l’administration de médicaments par voie orale. La plupart de ces répondants (92 % [12/13]) ont déclaré que les lignes directrices tenaient compte du temps écoulé après l’administration du médicament.
Conclusions :
Le problème des vomissements après l’administration de médicaments par voie orale était fréquent à cet hôpital et des lignes directrices sur la conduite à tenir dans ces cas étaient disponibles dans seulement quelques autres hôpitaux pour enfants. Les professionnels de la santé de cet établissement et d’autres établissements ont déclaré que le temps écoulé entre l’ingestion du médicament et le vomissement était le facteur le plus important dans la décision d’administrer une nouvelle dose du médicament.
MOTS CLÉS: vomissements , enfants , médicaments oraux , sondage
[Traduction par l’éditeur]
Children who have been admitted to hospital may vomit at some point during the hospital stay, as a result of a wide range of problems and illnesses, including acute gastroenteritis, adverse drug events, acute infection, and food poisoning.1 In addition, vomiting or regurgitation occurs frequently in otherwise well infants outside the hospital setting.1 The clinical significance of vomiting after administration of an oral medication varies, depending on the nature of the symptoms and disease being treated, the therapeutic index of the medication, the drug formulation (tablet, suspension, or sustained-release formulation), the pharmacokinetics of the drug, and the time between administration of the dose and emesis.
Very little information is available in the literature to suggest what action health care professionals should take in cases where a child vomits after administration of oral medication. Issues of 3 newsletters, the Canadian Pharmacist’s Letter ,2 the Nevada State Board of Pharmacy News ,3 and the newsletter of the Department of Pharmaceutical Care at the University of Iowa Hospitals and Clinics,4 have recently provided recommendations for redosing oral medications after vomiting. The consensus is to readminister the medication if it is visible in the vomitus or if vomiting has occurred within 15 min after ingestion of the dose.2–4 These newsletters emphasized that decisions to redose should be made on a case-by-case basis, taking into consideration the risk of undertreatment versus the risk of toxic effects, the therapeutic index of the medication, the type of medication (e.g., liquid, short-acting, long-acting), and the duration of treatment (i.e., short-course or long-term).2–4 However, none of the recommendations in these newsletters was specific to pediatric inpatients.
At the time of publication, in mid-2012, the Children’s and Women’s Health Centre of British Columbia (C&W), a tertiary care pediatric referral centre, had no formal policies in place to direct nurses, pharmacists, or physicians in the event of vomiting after ingestion of an oral medication, specifically with respect to whether the medication should be redosed. There appeared to be only a general consensus that medications should be readministered if emesis occurs within 15 min after ingestion of the dose and should not be readministered if emesis occurs more than 60 min after ingestion, with individual decisions made on a case-by-case basis. To determine the factors affecting clinical decisions about readministering medications after vomiting in pediatric patients, surveys of health care professionals at the authors’ institution and at other pediatric hospitals were undertaken. The specific objectives of the study were to characterize this clinical problem at C&W, as reported by health care professionals; to identify the current practice of medication redosing at C&W; and to collect guidelines and opinions from health care professionals at other pediatric hospitals regarding readministration of medications after vomiting.
Two voluntary, anonymous, self-administered online surveys were used to identify the current practices and opinions of nurses, pharmacists, and physicians working at pediatric hospitals, specifically whether to redose oral medications when vomiting occurs after their initial administration. The online survey software (SurveyMonkey) provided results as aggregated, de-identified data (i.e., not linked to a particular e-mail address or respondent). Approval from a research ethics board was not sought, nor was it required by the study institution, as the surveys did not involve patients or their families.
The first survey (see Appendix 1, available online at www.cjhp-online.ca/index.php/cjhp/issue/view/87/showToc) was specific to health care professionals practising at C&W and was conducted over a 1-month period (November to December 2010). An invitation to participate in the survey, with an embedded link to the online survey, was sent by e-mail (using institutional distribution lists) to potential pharmacy and nursing respondents; the number of invitations sent by this method is unknown. E-mail distribution lists for C&W physicians were not available to the investigators, so individual physicians (pediatricians and specialists) were contacted directly by e-mail with a request to participate. The survey for C&W staff members contained specific questions about current practice, as well as 6 clinical scenarios. Respondents were asked to rate the importance of the following 8 factors in the clinical decision to redose an oral medication after emesis: time after initial ingestion of dose, medication type, patient status, visibility of medication in vomitus, health care professional’s familiarity with the medication, dosage form, volume of vomitus, and patient age. Respondents used a 5-point Likert scale (where 1 = not important, 3 = somewhat important, and 5 = very important) to rate the factors. The 6 clinical scenarios presented details about the patient’s age, the medication and dosage regimen, the time to emesis, and the visibility of the medication in the vomitus. One scenario served as a “baseline”, with one factor being altered in each of the other scenarios. Respondents were asked whether they had encountered a similar situation in the past, whether they would readminister the medication, and if so, why they would take that action. The scenarios were intended to elicit more information about the influence of these factors in a simulated clinical setting.
The second survey (Appendix 2, available online at www.cjhp-online.ca/index.php/cjhp/issue/view/87/showToc) was open (for a 1-month period, from November to December 2010) to health care professionals at other Canadian and US pediatric hospitals, as well as to pediatric pharmacists practising in adult hospitals. The survey was posted on professional health care forums, including those of the Canadian Society of Hospital Pharmacists, the American College of Clinical Pharmacy, mmp|Bench, the Chemotherapy and Biotherapy Instructors of the Association of Pediatric Hematology/Oncology Nurses, and the Canadian Oncology Nurse Educators Group. This survey asked questions about the presence of guidelines and recommendations, as well as perceived practice, at the respondent’s hospital. If a guideline was available, the survey requested information about the characteristics of the guideline, which of the 8 possible factors (listed above) were taken into account by the guideline, and any references used in development of the guideline. If no guideline was available, information about common practice at the respondent’s hospital was requested by means of open-ended questions.
A total of 76 responses were received from pharmacists, nurses, and physicians at C&W, of which 11 were incomplete except for demographic data and were therefore excluded. The remaining 65 responses were included in the analysis. Nurses accounted for most of the C&W respondents, and most respondents were working in the area of general pediatrics (Table 1).
Table 1.
Characteristics of Respondents to Survey of Health Care Professionals at Children’s and Women’s Hospital of British Columbia

The majority of respondents indicated that they had encountered the problem of vomiting after administration of oral medications either weekly (25/65 [38%]) or monthly (24/65 [37%]) in the past 6 months. Most of the other respondents (15/65 [23%]) had never encountered the problem, and only 1 respondent (2%) encountered it daily. The problem was most frequently seen in patients 1 to 4 years of age (22/50 [44%]) or less than 1 year of age (13/50 [26%]).
Most respondents reported that in cases of vomiting after oral administration of medications, they would redose the medication according to a general rule based on the time elapsed between ingestion and vomiting: either within 30 min (39/65 [60%]) or within 15 min (21/65 [32%]). The remaining 5 (8%) of respondents indicated that they would not follow a general rule, and no respondents indicated following a general rule to redose if vomiting occurred within 1 h after ingestion.
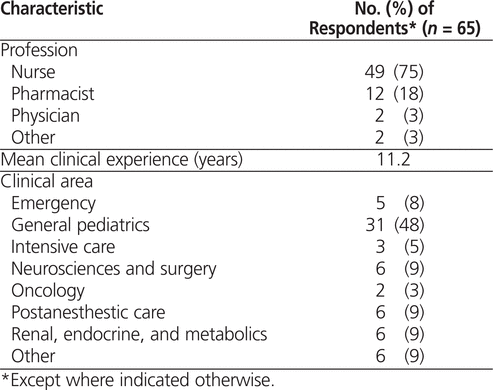
Respondents rated the importance of 8 factors affecting the clinical decision to redose oral medications after emesis on a 5-point Likert scale, and 4 of these factors were given a rating of 5 (indicating “very important”) by more than half of respondents: time after dose ingestion, medication type, patient status, and visibility of medication in vomitus (Table 2). For all 4 of these factors, the mean importance rating was greater than 4 (Table 2).
Table 2.
Importance of Factors Affecting Decision to Redose an Oral Medication after Vomiting among Health Care Professionals at Children’s and Women’s Hospital of British Columbia
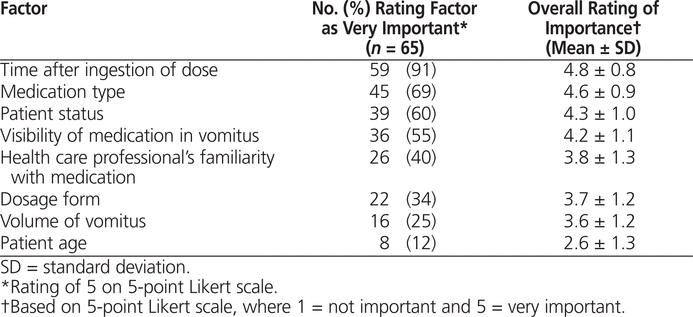
More than half of the respondents had encountered situations in practice that were similar to the first 5 of the 6 clinical scenarios described in the survey (Table 3), but only 12 (21%) of 57 respondents had encountered a situation similar to scenario 6. For each of the 6 scenarios, at least 60% of respondents had the same clinical decision about redosing the medication (Table 3). Of the factors potentially affecting the decision to redose, time after dose ingestion was most often taken into consideration, whereas the patient’s age was least often taken into consideration. The extent to which the type of medication affected the clinical decision varied greatly among the 6 clinical scenarios. Most respondents considered this factor for scenario 6, in which the medication was long-acting morphine.
Table 3.
Characteristics of and Responses to 6 Clinical Scenarios Presented in Survey of Health Care Professionals at Children’s and Women’s Hospital of British Columbia
A total of 53 responses were received from other institutions, of which 47 were complete. The 6 partially completed responses were included in the analysis.
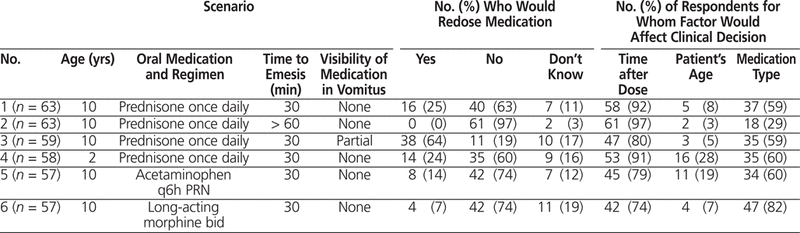
Sixteen (30%) of the respondents indicated that their pediatric hospital or ward had a guideline, protocol, and/or recommendation regarding what to do in the event of vomiting after ingestion of oral medications. The majority of these guidelines were ward- or service-specific (9 [56%]); 3 (19%) were hospital-wide, and 2 (12%) were drug-specific. Thirteen of the respondents provided further details about their respective guidelines (Table 4). Most stated that their guidelines accounted for the time after dose ingestion; other factors were variably considered. None of the guidelines took into account the patient’s status or the health care provider’s familiarity with the medication. Of the guidelines used in respondents’ institutions, only 3 had supporting references: 1 guideline was based on an oncology-specific recommendation for practice,5 and 2 guidelines were based on the article in Canadian Pharmacist’s Letter .2 Only 2 respondents submitted their institutions’ guidelines along with the survey response; in both cases, the guideline consisted of the article in the Canadian Pharmacist’s Letter. 2
Table 4.
Factors Addressed in Guidelines of Other Pediatric Institutions, as Reported by Survey Respondents
Thirty-seven (70%) of the 53 respondents reported that their pediatric health facility or unit did not have any guidelines, protocols, or recommendations regarding what to do in the event of vomiting after ingestion of oral medications. Of these, the majority (22 [59%]) indicated that the dose would be repeated if vomiting occurred within a specific time frame. Other respondents indicated that their decision would be based on professional judgment (19 [51%]) or that they would contact the prescriber (11 [30%]).
The problem of vomiting after administration of oral medications in children was prevalent at the authors’ institution, with 75% of respondents reporting that they encountered the problem either weekly or monthly. Despite the prevalence of this problem, there is a lack of studies and evidence-based recommendations as to the best course of action (specifically, when to redose the medication). The most comprehensive published recommendation, found in the Canadian Pharmacist’s Letter and based on expert opinion, states that the tablet or capsule can be readministered if it is visible in the vomitus or if vomiting occurred within 15 min of ingestion.2 The recommendation also states that the decision should be made on a case-by-case basis, taking into account the potential risk of missing a dose.2
In designing the surveys used in this study, we were interested in the factors influencing health care professionals’ decisions on the course of action to be taken when a pediatric inpatient vomits a dose of oral medication. We asked about factors that were considered in the published recommendations that we identified,2–4 as well as factors that we thought might be specific to pediatric patients or to hospital inpatients. In the survey of current practice at C&W, the most important factors that health care professionals used to decide about redosing were time after ingestion, medication type, patient status, and visibility of medication in the vomitus. The caregiver’s familiarity with the medication, the dosage form, and the volume of vomitus were less important, and the patient’s age was minimally important. Interestingly, in the survey of health care professionals at other pediatric institutions and pediatric pharmacists, the guidelines, if present, did not consider patient status or caregiver’s familiarity with the medication. However, most of the guidelines considered time after dose ingestion, and about one-third considered medication type and visibility of medication in the vomitus.
The difficulty in making a specific recommendation on the best course of action after a pediatric inpatient has vomited a dose of medication likely stems from the diverse nature of the patients, their illnesses, and the medications themselves. Many health care professionals at the authors’ institution, as well as those at other pediatric hospitals, reported following a general rule based on the time from dose administration to vomiting in deciding whether to readminister a medication, but this approach may not always be appropriate. For example, redosing a liquid medication if vomiting occurs within 30 min after ingestion may not be appropriate for all patients, because there is a lack of information about gastrointestinal transit time and gastric emptying time for various dosage forms in children.6 In particular, it is thought that the gastric emptying time for milk is 30 to 120 min, depending on the age of the child and the type of milk.6
Among health care professionals who stated that they followed a general rule based on timing of vomiting relative to dose administration, clinical judgment also appeared to be used. Medication type was considered the second most important factor in a decision to redose a vomited medication. The indication for the medication, the therapeutic window, the pharmacokinetics and pharmacodynamics of the drug, potential adverse effects, and dosing interval are all factors that could be considered under the general topic of “medication type”.
The information collected in this study was subject to the limitations inherent to survey studies, such as the potential for recall bias, as well as limitations specific to the design and distribution method for these particular surveys. The surveys were not validated or tested before use in the study. The information collected in both surveys was limited because we did not always allow respondents to provide a written answer, prompting them instead with check boxes. The 6 scenarios were closely interrelated (each varying in one respect from a baseline scenario), which may have led respondents to answer differently than they would behave in practice had each scenario been an independent occurrence. The survey of C&W staff had few physician respondents, which was likely due, at least in part, to the lack of availability of an e-mail distribution list for physicians. This limitation may have led to selection bias. For the survey of other pediatric hospitals and pediatric pharmacists, the survey was posted to various health care forums, rather than being sent directly to individual pediatric hospitals. We focused primarily on forums for pediatric pharmacists and oncology nurses, as these were the forums to which we had access. Not using general pediatric nursing forums or pediatric physician forums likely led to further selection bias. We do not know whether there were multiple responses from a single institution, nor do we know how many and which institutions were not represented by respondents. We also do not know if respondents from institutions that had ward- or medication-specific guidelines were necessarily aware of those guidelines. Some respondents did not answer all questions, which is a common problem in survey studies. In addition, because of our reliance primarily on e-mail distribution lists and health care forums, we do not know how many invitations to participate were sent, and we are therefore unable to calculate a response rate for either survey.
For the survey of other hospitals, respondents reported that they would typically contact the prescriber in the case of a vomited dose. Therefore, a next step in studying this problem might be to survey prescribers directly to determine what factors they consider in deciding whether a medication should be redosed. It may also be helpful to ask prescribers and health care professionals about perceived knowledge gaps and the information or training that would help them in making the decision to redose a medication.
More than three-quarters of health care professionals responding to a survey at the authors’ institution reported encountering the problem of vomiting after administration of an oral medication either weekly or monthly. This problem was reported to occur most often in children 4 years of age or younger. Among health care professionals at the authors’ institution, the elapsed time between dose ingestion and vomiting appeared to be the most important factor in the decision on whether or not to readminister the medication; however, the elapsed time used in this decision was not consistent among respondents. Health care professionals at this institution also listed medication type, patient status, and visibility of medication in the vomitus as important factors in their decision.
Less than one-third of health care professionals from other institutions who responded to the survey reported that their institution had a guideline providing direction in this situation. The factors most often addressed in these guidelines were time between dose ingestion and vomiting, medication type, and visibility of medication in the vomitus. In the absence of a guideline at their respective hospitals, health care professionals reported that their decision on whether to redose a medication after vomiting was based on a specific elapsed time or on their professional judgment.
The risk of therapeutic failure if a dose is not readministered but has not been absorbed (because of vomiting) must be weighed against the risk of toxic effects should the dose be readministered when in fact it was absorbed (before vomiting occurred). This decision should probably be made on a case-by-case basis for pediatric inpatients; however, health care professionals may find it helpful to have a decision-making algorithm or a list of factors to consider. We plan to provide education for health care professionals and to create a guidance document for use at our pediatric institution, outlining the factors to consider when deciding the best course of action for patients who have vomited a dose of medication.
1. Allen K. The vomiting child—what to do and when to consult. Aust Fam Phys 2007;36(9):684–687.
2. O’Mara NB. Redosing of selected medications after vomiting. Can Pharm Lett 2009 [cited 2010 Sep 27];25(9):250909. Available from: http://canadianpharmacistsletter.therapeuticresearch.com/pl/DD_pu.aspx?cs=&s=PLC&pt=3&dd=250909 (subscription required to access content)
3. The vomiting patient. Nevada State Bd Pharm News 2010 [cited 2010 Sep 27];21(2):1,4. Available from: http://bop.nv.gov/Newsletters/2010/2010-04_Newsletter_bop.pdf
4. Re-dosing medications if the patient vomits. In: Murhammer J, Ross M, Bebout K, editors. Rx update . Iowa City (IA): University of Iowa Hospitals and Clinics, Department of Pharmaceutical Care; 2009 Oct [cited 2010 Sep 27]. Available from: www.healthcare.uiowa.edu/pharmacy/RxUpdate/2009/October09.pdf
5. Povlovich M, Whitford J, Olsen M. Chemotherapy and biotherapy guidelines and recommendations for practice. 3rd ed. Pittsburgh (PA): Oncology Nursing Society; 2009.
6.
Bowles A, Keane J, Ernest T, Clapham D, Tuleu C. Specific aspects of gastro-intestinal transit time in children for drug delivery design.
Int J Pharm
2010;395(1–2):37–43.
Canadian Journal of Hospital Pharmacy , VOLUME 65 , NUMBER 3 , May-June 2012