Steven J Celetti , Régis Vaillancourt , Elena Pascuet , Diane SharpABSTRACT
Background:
At the Children’s Hospital of Eastern Ontario, more than 6000 inpatients per year undergo IV saline flushes by prefilled syringe to assess and maintain patency of IV tubing. In studies involving adults, it has been reported that volatile substances may leach from syringe materials into the saline, leading to taste and/or odour disturbances.
Objective:
To determine the incidence of taste and/or odour disturbances in pediatric patients after flushing of IV tubing with 0.9% sodium chloride (normal saline [NS]) from prefilled syringes.
Methods:
Inpatients aged 5–18 years who had undergone routine flushing of central or peripheral IV tubing with commercially available prefilled NS syringes were interviewed. Children aged 5–10 years used a visual hedonic scale to rate taste and odour sensations, and those aged 11–18 years used a numeric rating scale.
Results:
During the study period (April to July 2011), a total of 104 pediatric inpatients (21 aged 5–10 years and 83 aged 11–18 years) underwent NS flushing of central (10 patients [10%]) or peripheral (94 patients [90%]) tubing. For 100 of these patients, BD Posiflush NaCl 0.9% 10-mL sterile prefilled syringes were used, and for 4 patients BD Saline XS NaCl 0.9% 10-mL sterile prefilled syringes were used. Taste and/or odour disturbances were reported by 76 (73%) of the patients. Twelve patients described more than one taste or odour sensation. Taste and odour disturbances were detected by children in both age groups.
Conclusions:
Flushing of IV tubing with prefilled NS syringes resulted in taste and/or odour disturbances in a pediatric population.
KEYWORDS: taste and odour disturbances , administration of IV saline flush , prefilled syringes
RÉSUMÉ
Contexte :
Au Centre hospitalier pour enfants de l’est de l’Ontario, on procède chez plus de 6 000 patients hospitalisés par année au rinçage de leur tubulure i.v. au moyen de seringues préremplies d’une solution de chlorure de sodium à 0,9 % (solution physiologique salée [SP]) afin d’évaluer et de maintenir la perméabilité des tubulures. Lors d’études chez des patients adultes, on a signalé que des substances volatiles pourraient s’échapper du matériel composant la seringue et passer dans la SP, entraînant des altérations du goût ou de l’odorat.
Objectif :
Déterminer l’incidence des épisodes d’altération du goût ou de l’odorat chez des enfants hospitalisés dont la tubulure i.v. a été rincée à l’aide d’une seringue préremplie de SP.
Méthodes :
Des patients hospitalisés âgés de 5 à 18 ans chez qui on a procédé au rinçage habituel de leur tubulure i.v. périphérique ou centrale à l’aide de seringues commerciales préremplies de SP ont été interrogés. Les enfants âgés de 5 à 10 ans ont utilisé une échelle visuelle hédonique pour évaluer les sensations gustatives et olfactives et ceux âgés de 11 à 18 ans ont utilisé une échelle d’évaluation numérique.
Résultats :
Au cours de la période de l’étude (avril à juillet 2011), on a procédé chez 104 enfants hospitalisés (21 âgés de 5 à 10 ans et 83 âgés de 11 à 18 ans) au moyen des seringues de SP au rinçage de leur tubulure centrale (10 patients [10 %]) ou périphérique (94 patients [90 %]). On a utilisé chez 100 de ces patients une seringue préremplie stérile BD Posiflush de 10 mL et chez quatre patients, une seringue préremplie stérile BD Saline XS de 10 mL. Des altérations du goût ou de l’odorat ont été signalées par 76 (73 %) patients. Douze patients ont décrit plus d’une sensation gustative ou olfactive. Des altérations du goût ou de l’odorat ont été notées dans les deux groupes d’âge.
Conclusions :
Le rinçage de la tubulure i.v. au moyen de seringues préremplies de SP a entraîné des altérations du goût ou de l’odorat dans cette population d’enfants hospitalisés.
MOTS CLÉS: altérations du goût et de l’odorat , administration de solution salée i.v. pour le rinçage , seringues préremplies
[Traduction par l’éditeur]
IV tubing is regularly flushed with 0.9% sodium chloride (normal saline [NS]) to assess and maintain patency. In a recent study, 10 healthy adult volunteers experienced unpleasant taste and/or odour sensations in the mouth after injection of NS from commercially available prefilled syringes.1 The syringe manufacturer, BD (Franklin Lakes, New Jersey), identified volatile, nontoxic substances (i.e., 2-methyl-2-propanol, 2-methyl-2-butanol, and ethyl-butyl-ether) by high-performance liquid chromatography.1 Kongsgaard and others1 postulated that these substances would be detected by the patient’s olfactory system, resulting in taste and odour disturbances when eliminated via the respiratory system.
The Children’s Hospital of Eastern Ontario provides care for over 6000 inpatients each year. Almost all of these patients will undergo NS flushing of IV tubing from a prefilled syringe at least once during their hospital stay. Nurses on the Vascular Access Team have sole responsibility for IV flushing and have anecdotally reported findings similar to those of Kongsgaard and others.1 It was thought that these anecdotal reports warranted further investigation. To the authors’ knowledge, there has to date been no description in the literature of the incidence of taste and odour experiences related to flushing of IV tubing among pediatric patients. Altered sensations of this type may be especially bothersome for patients undergoing chemotherapy or radiotherapy, since these treatments have also been shown to alter taste and/or odour perceptions.2 In addition, IV pentamidine, which is used as first-line prophylactic therapy for Pneumocystis carinii pneumonia in patients with AIDS, was directly associated with altered taste sensations.3
This observational study was conducted to determine the incidence of taste and/or odour experiences among pediatric patients, secondary to administration of IV flushing with NS from prefilled syringes. In addition, the authors sought to determine whether a correlation exists between patients’ taste and/or odour perceptions and the volume and/or rate of administration of NS from the prefilled syringe.
This prospective observational study was approved by the CHEO Research Ethics Board and was conducted from April 4, 2011, to July 21, 2011, exclusively at the Children’s Hospital of Eastern Ontario, a tertiary care teaching facility in Ottawa, Ontario. Consecutive inpatients aged 5 to 18 years for whom routine flushing of central or peripheral IV tubing was to be performed (as part of their usual care) with BD Saline XS NaCl 0.9% 10-mL sterile prefilled syringe and/or BD Posiflush NaCl 0.9% 10-mL sterile prefilled syringe and who met the inclusion criteria were invited to participate in the study. The following groups of patients were excluded: children with known developmental delays, as recorded in the patients’ charts; children for whom the task would have been uncomfortable, in the clinical estimation of the Vascular Access Team nurse; children with documentation of recent head trauma, abdominal pain, rhinorrhea, or cough; and children who did not speak or understand English or French ((or children whose parents or guardian did not understand English or French), because of the importance of fully understanding the questions being asked.
A Vascular Access Team nurse administered NS into IV tubing, from prefilled syringes, according to usual hospital protocol, for any patients requiring this procedure. The nurse explained that he or she wanted to ask a few questions specifically related to this study. Verbal consent was obtained by informing each eligible pediatric patient that an assessment of the smell and taste of the prefilled NS products was being conducted and by asking the patient (or a parent or guardian) to agree to participate. Immediately after the flushing procedure, the nurse interviewed the patient and collected all study-related information and patient responses on data collection sheets. The following data were collected: date of birth, sex, date of flushing procedure, location of tubing flushed (central or peripheral), type of syringe (according to model name), volume and rate of administration, and taste and odour perceptions (as both descriptions and ratings). Children aged 5–10 years were asked to rate any taste and/or odour on a validated visual hedonic scale, where 0 indicated the worst taste or odour and 100 represented the best taste or odour.4–9 Children aged 11–18 years were asked to assess taste and/or odour on a numeric rating scale, where 0 represented no bad taste or odour and 10 represented the worst taste or odour conceivable, consistent with other scales used in studies of taste and/or odour disturbance.1
Descriptive statistics were calculated on the incidence of taste and/or odour deviations secondary to administration of NS flushing of IV tubing by prefilled syringe. Categorical variables were summarized using frequencies and percentages. Data were analyzed with SPSS software, version 18.0 (SPSS Inc, Chicago, Illinois). Statistical significance was determined using χ2, Fisher’s exact, Breslow–Day, and/or Mantel–Haenszel tests as appropriate. Analysis was completed for the following measurable outcomes: total number of reported deviations in taste and/or odour (overall incidence rate); variation in incidence based on age or sex of the patient; most common descriptions of reported alterations in taste and/or odour; and correlations between the patient’s taste and/or odour perception and the volume and/or rate of administration of the NS flush from the prefilled syringe. Any p values less than 0.05 were deemed statistically significant. Descriptive statistics are reported as mean ± standard deviation.
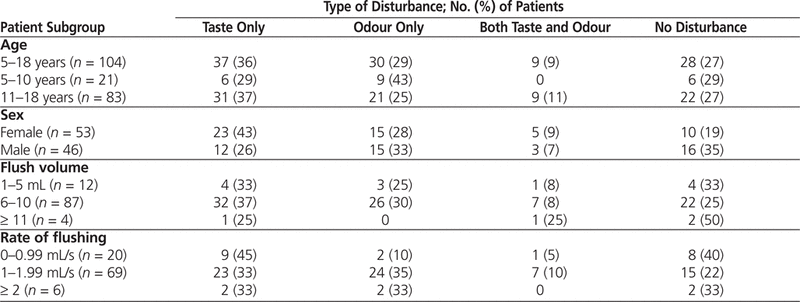
All 107 pediatric patients approached gave their consent and were interviewed. Three of the patients were excluded because of missing information (i.e., date of birth), and data for 104 patients were analyzed. Taste and/or odour disturbances were reported by 76 (73%) of the patients aged 5–18 years who had received NS flush of IV tubing. Taste and/or odour disturbances were reported by 81% (43/53) of the female patients and 65% (30/46) of the male patients ( p = 0.11). There were no significant differences in taste and/or odour disturbances among other patient subgroups defined on the basis of age, flush volume, or rate of flushing (Table 1).
Table 1.
Incidence of Taste and/or Odour Disturbances

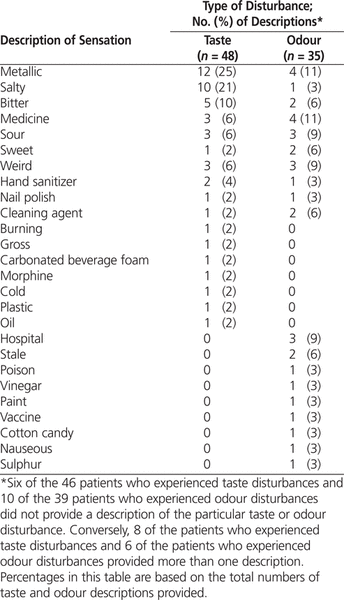
The most frequently reported tastes were metallic (12/48 [25%]) and salty (10/48 [21%]) (Table 2). Eight of the patients who described a taste disturbance reported more than one such altered sensation (Table 2). The mean taste disturbance among older patients, based on the numeric rating scale (from 0 to 10), was 5.1 ± 2.3 ( n = 40) and among younger patients, based on the visual hedonic scale (from 0 to 100), was 56.3 ± 31.5 ( n = 4). The most frequently reported odours were metallic and “like medicine” (4/35 [11%] for both). Six of the patients who described an odour disturbance reported more than one such altered sensation (Table 2). The mean odour disturbance among older patients, based on the numeric rating scale (from 0 to 10), was 5.0 ± 1.5 ( n = 29) and among younger patients, based on the visual hedonic scale (from 0 to 100), was 40 ± 12.2 ( n = 6).
Table 2.
Frequency and Description of Taste and Odour Disturbances
Most of the patients ( n = 100) underwent flushing with BD Posiflush syringes, whereas 4 received flushing with BD Saline XS syringes. Ninety-four patients underwent flushing of peripheral tubing, and 10 underwent flushing of central tubing. The most frequently administered volume was 10 mL (for 62 of 103 patients), and the most frequently used rate of administration was 1 mL/s (for 51 of 95 patients).
It is common practice, including at the Children’s Hospital of Eastern Ontario, to maintain patency of IV tubing by NS flushing.10,11 Using a numeric rating scale and a visual hedonic scale, we found that modest disturbances in taste and/or odour occurred in the pediatric population following NS flushing of IV tubing from prefilled syringes. The minimum age for inclusion in the current study was set at 5 years, because previous studies have demonstrated the ability of children as young as 5 years to reliably evaluate palatability.4–9 The visual hedonic scale used here was previously used in taste studies of antibiotics in children.4–9
There were no obvious correlations between the subgroups studied (based on age, sex, and volume or rate of flushing), so these taste and/or odour sensations could occur in any patient. Identifying and preventing additional sources of alteration in taste and/or odour is especially important for patients receiving chemotherapy or radiotherapy, since these treatments may directly alter taste and odour perceptions, including the experience of metallic or bitter sensations.2 These disturbances in taste and/or odour perceptions may decrease patients’ quality of life and may lead to malnutrition and significant morbidity.12,13
In agreement with findings reported by Kongsgaard and others,1 who also used BD prefilled NS syringes, a high incidence of taste and/or odour disturbances was observed in this study among patients who underwent NS flushing of IV tubing. As reported by the authors of the earlier study,1 the manufacturer noted that volatile substances leaching into the saline from the plastic syringes are linked to patients’ experience of bad tastes or smells, but are not harmful to health. In addition, a smaller volume or slower flush rate did not appear to affect the incidence of taste and/or odour disturbance in the current study, in contrast to the manufacturer’s claims (as reported by Kongsgaard and others1).
Just over a decade ago, unidentified compounds leached into prefilled syringes and ultimately led to adverse patient outcomes. Patients treated with epoetin alfa administered from prefilled syringes were found to have leached aromatic compounds in their bodies. These compounds led to the occurrence of adverse events, including antibody-positive pure red cell aplasia.14 Since then, modifications to the syringe-manufacturing process have been made to allow prospective identification of substances that may leach into prefilled syringes and cause deleterious effects on patients’ health.15
In recent years, the use of prefilled syringes has increased,16 and such syringes have become widely adopted in the self-administration of medications, such as insulin for treating diabetes mellitus.17 There are several potential or proven advantages to using prefilled syringes, including improved accuracy of dosing,18 reduced risk of dosing and medication errors, reduced risk of microbial contamination, less need for overfilling and hence less wastage, and increased safety and convenience for emergency use.19 However, the lack of data about compounds that leach into the contents of prefilled syringes suggests that reporting of adverse events associated with prefilled syringes needs to be improved.
One limitation of this study was the use of 2 different scales for the reporting of taste and/or odour disturbance. The validated visual hedonic scale has been used in several palatability studies,4–9 including studies in children aged 5 to 10 years,4 but the numeric scale used for children aged 11 to 18 years has not yet been validated. However, it appears that the modest alterations in intensity of taste and/or odour sensations observed in this study were similar to those reported by Kongsgaard and others,1 which suggests that it may be worthwhile to validate this numeric scale in future taste and/or odour studies involving children aged 11 to 18 years and adults.
The lack of a control group for comparison with patients receiving NS flush via prefilled syringes indicates the potential for taste and/or odour disturbances among hospital inpatients more generally. Potential sources that may contribute to abnormal taste and/or odour sensations include IV Viaflex bags (Baxter), tubing, or the parenteral medications themselves. These potential sources remain to be investigated. Another limitation of this study was the involvement of several different nurses, which may have prevented a standardized method of interviewing patients or introduced bias. Furthermore, the nurses were required to ask patients directly about possible taste or odour disturbances experienced, as a part of the interview process, rather than obtaining the information in some more objective manner.
There exists a risk of over-reporting taste and/or odour disturbances caused by NS flush with prefilled syringes. However, this study was initiated in response to spontaneous reports from pediatric patients who were able to independently link the abnormal sensations to flushing with a prefilled syringe (anecdotal evidence). The options to address this patient concern include replacing the syringes with syringes that do not leak unidentified compounds, asking the manufacturer to address the problem, or using syringes that have not been prefilled. Manufacturers should perform more vigilant quality control, using chromatographic and/or spectroscopic methods, to identify alterations in the long-term composition of prefilled syringe products.20,21
Use of control prefilled syringes manufactured by another company could also have improved the strength of the analysis. However, there are no reports that prefilled NS syringes sold by other manufacturers have been linked to taste and/or odour disturbances. Patients with abdominal pain, rhinorrhea, and/or cough were excluded from the study, because upper respiratory and gastrointestinal tract symptoms have been shown to alter taste perception.4 Bias was reduced by obtaining formal consent after routine administration of the NS flush, since patients may be more likely to report taste and/or smell anomalies when specifically told that such anomalies are the subject of the investigation.
Future studies could investigate potential taste and/or odour disturbances related to IV medications compounded in NS using BD syringes, since pediatric hospitals using this brand of syringe may store such medications for up to 30 days. Clinical outcomes were not assessed in the current study, but further investigation could be directed at assessing patients’ willingness to undergo subsequent NS flush after reporting altered taste and/or odour sensations and the effect of such altered sensations on inpatient quality of care. To improve inpatient quality of life, future studies should include blinding of the research team and should aim to compare different brands of prefilled syringes with syringes prepared fresh in the pharmacy.
1.
Kongsgaard UE, Andersen A, Oien M, Oswald IA, Bruun LI. Experience of unpleasant sensations in the mouth after injection of saline from prefilled syringes.
BMC Nurs
2010;9:1–6.

2.
Hong JH, Omur-Ozbek P, Stanek BT, Dietrich AM, Duncan SE, Lee YW, et al. Taste and odor abnormalities in cancer patients.
J Support Oncol
2009;7(2):58–65.
3.
Glover J, Dibble S, Miaskowski C, Geibert R. Changes in taste associated with intravenous administration of pentamidine (revised).
J Assoc Nurses AIDS Care
1995;6(2):41–46.
4.
Freedman SB, Cho D, Boutis K, Stephens D, Schuh S. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial.
Arch Pediatr Adolesc Med
2010;164(8):696–702.
5.
Matsui D, Barron A, Rieder MJ. Assessment of the palatability of antistaphylococcal antibiotics in pediatric volunteers.
Ann Pharmacother
1996;30(6):586–588.
6.
Cheng A, Ratnapalan S. Improving the palatability of activated charcoal in pediatric patients.
Pediatr Emerg Care
2007;23(6):384–386.
7.
Sjövall J, Fogh A, Huitfeldt B, Karlsson G, Nylén O. Methods for evaluating the taste of paediatric formulations in children: a comparison between the facial hedonic method and the patients’ own spontaneous verbal judgement.
Eur J Pediatr
1984;141(4):243–247.
8.
Angelilli ML, Toscani M, Matsui DM, Rieder MJ. Palatability of oral antibiotics among children in an urban primary care center.
Arch Pediatr Adolesc Med
2000;154(3):267–270.
9.
Dagnone D, Matsui D, Rieder MJ. Assessment of the palatability of vehicles for activated charcoal in pediatric volunteers.
Pediatr Emerg Care
2002;18(1):19–21.
10.
Kotter RW. Heparin vs saline for intermittent intravenous device maintenance in neonates.
Neonatal Netw
1996;15(6):43–47.
11.
Hamilton RA, Plis JM, Clay C, Sylvan L. Heparin sodium versus 0.9% sodium chloride injection for maintaining patency of indwelling intermittent infusion devices.
Clin Pharm
1988;7(6):439–443.
12.
DeWys WD, Walters K. Abnormalities of taste sensation in cancer patients.
Cancer
1975;36(5):1888–1896.
13.
Pattison RM, Dougan H, Richardson RA, Davidson HIM. Biochemical correlates of altered taste perception in patients with advanced cancer [abstract].
Clin Nutr
1997;16(2 Suppl):S29.
14. Pang J, Blanc T, Brown J, Labrenz S, Villalobos A, Depaolis A, et al. Recognition and identification of UV-absorbing leachables in EPREX® pre-filled syringes: an unexpected occurrence at a formulation–component interface. PDA J Pharm Sci Technol 2007;61(6):423–432.
15.
Bennett CL, Luminari S, Nissenson AR, Tallman MS, Klinge SA, McWilliams N, et al. Pure red-cell aplasia and epoetin therapy.
N Engl J Med
2004;351(14):1403–1408.
16.
Rathorne N, Pranay P, Eu B, Ji W, Walls E. Variability in syringe components and its impact on functionality of delivery systems.
PDA J Pharm Sci Technol
2011;65(5):468–480.
17.
Korytkowski M, Bell D, Jacobsen C, Suwannasari R; FlexPen Study Team. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus.
Clin Ther
2003;25(11):2836–2848.
18.
Wielandt JO, Niemeyer M, Hansen MR, Bucher D, Thomsen NB. An assessment of dose accuracy and injection force of a novel prefilled insulin pen: comparison with a widely used prefilled insulin pen.
Expert Opin Drug Deliv
2011;8(10):1271–1276.
19.
Webster CS, Merry AF, Gander PH, Mann NK. A prospective, randomised clinical evaluation of a new safety-orientated injectable drug administration system in comparison with conventional methods.
Anaesthesia
2004; 59(1):80–87.
20.
Koppel C, Tenczer J. Scope and limitations of a general unknown screening by gas chromatography – mass spectrometry in acute poisoning.
J Am Soc Mass Spectrom
1995;6(11):995–1003.
21.
Marquet P. Is LC-MS suitable for a comprehensive screening of drugs and poisons in clinical toxicology?
Ther Drug Monit
2002;24:125–133.
The authors thank Aman Hansra for her collaboration with the initial research project.
Canadian Journal of Hospital Pharmacy , VOLUME 65 , NUMBER 5 , September-October 2012