Claudia K Ho , Vincent H Mabasa , Vivian W Y Leung , Douglas L Malyuk , Jerrold L PerrottABSTRACT
Background
Clinical pharmacy services have been shown to reduce adverse drug events and health care costs. However, few studies have assessed their effect on patient outcomes in the intensive care unit (ICU).
Objective:
To describe characteristics of ICU patients with documented pharmacist interventions and to evaluate the relationships between patients’ complexity level and pharmacists’ interventions and between pharmacists’ interventions and mortality rate.
Methods:
Inpatient records of admissions between January 1, 2004, and March 31, 2007, were analyzed to identify the presence of clinical pharmacy notes (CPNs). The characteristics of patients with and without CPNs were compared using descriptive statistics. For primary analysis of the association between patient complexity level and presence of CPNs, logistic regression modelling was performed to adjust for potential confounding. Logistic regression was also used to explore the possible association between CPNs and mortality. Finally, mortality analysis was carried out for patients with and without CPNs, with matching by complexity level.
Results:
The main study cohort comprised 1561 patients: 333 (21.3%) with CPNs and 1228 (78.7%) with no CPNs. A greater proportion of those with a CPN had the highest complexity level: 295 (88.6%) of those with CPNs versus 660 (53.7%) of those with no CPNs. After adjustment for age and sex, the odds ratio for having a CPN among patients with complexity level 4 (relative to patients with lower complexity levels) was 8.20 (95% confidence interval 5.44–12.38). Mortality rates were not significantly different between the 2 groups: 26.7% (89/333) among patients with CPNs and 27.9% (343/1228) among those without CPNs ( p = 0.66). After adjustment for age, sex, complexity level, and length of stay in the ICU, the presence of a CPN was not significantly associated with mortality. Mortality rates in the matched cohort ( n = 1078) were also similar between patients with and without CPNs (89/333 [26.7%] and 226/745 [30.3%], respectively; p = 0.23), and the presence of a CPN was not significantly associated with mortality after adjustments for potential confounding factors.
Conclusion:
Documenting clinical pharmacy activities is essential for assessing pharmacists’ impact on patient outcomes. These data suggest that ICU pharmacists prioritize clinical activities to care for the sickest patients.
KEYWORDS: clinical pharmacy , documentation , patient outcomes , interventions , mortality , length of stay
RÉSUMÉ
Contexte :
Il a été montré que les services de pharmacie clinique réduisaient les événements indésirables liés aux médicaments et les coûts de soins de santé. En revanche, peu d’études ont évalué leurs effets sur les résultats thérapeutiques chez les patients des unités de soins intensifs (USI).
Objectif :
Décrire les caractéristiques des patients des USI pour lesquels les pharmaciens avaient consignés des interventions et évaluer les liens entre le niveau de complexité de l’état des patients et les interventions des pharmaciens et entre les interventions des pharmaciens et le taux de mortalité.
Méthodes :
Les dossiers des patients hospitalisés entre le 1er janvier 2004 et le 31 mars 2007 ont été analysés à la recherche de notes de pharmaciens cliniciens (NPC). Les caractéristiques des patients dont le dossier comportait des NPC et de ceux dont le dossier n’en comportait pas ont été comparées au moyen de statistiques descriptives. L’analyse primaire de l’association entre le niveau de complexité de l’état des patients et la présence de NPC a été réalisée au moyen d’un modèle de régression logistique pour compenser les facteurs de confusion potentiels. Ce modèle a aussi été utilisé pour évaluer l’association possible entre les NPC et la mortalité. En dernier lieu, une analyse de mortalité a comparé les patients dont le dossier comportait des NPC à ceux dont le dossier n’en comportait pas, avec un appariement du niveau de complexité.
Résultats :
La principale cohorte de l’étude comptait 1561 patients : 333 (21,3 %) dont le dossier comportait des NPC et 1228 (78,7 %) dont le dossier n’en comportait pas. Une plus grande proportion des patients dont le dossier comportait des NPC présentaient le plus haut niveau de complexité : 295 (88,6 %) de ceux avec des NPC contre 660 (53,7 %) de ceux sans NPC. Après ajustement pour l’âge et le sexe, le risque relatif approché de NPC chez les patients présentant un niveau de complexité 4 (par rapport aux patients présentant un niveau de complexité moindre) était de 8,20 (intervalle de confiance à 95 % : 5,44 – 12,38). Les taux de mortalité n’étaient pas significativement différents entre les deux groupes : 26,7 % (89/333) chez les patients avec NPC et 27,9 % (343/1228) chez les patients sans NPC ( p = 0,66). Après ajustement pour l’âge, le sexe, le niveau de complexité et la durée du séjour à l’USI, la présence d’une NPC au dossier n’était pas associée de façon significative à la mortalité. Les taux de mortalité au sein de la cohorte appariée ( n = 1078) étaient également similaires entre les patients avec NPC et ceux sans NPC : respectivement 89/333 (26,7 %) et 226/745 (30,3 %) ( p = 0,23); la présence d’une NPC n’a pas été associée de façon significative à la mortalité après ajustements pour les facteurs de confusion potentiels.
Conclusion :
La consignation des activités de pharmacie clinique est essentielle à l’évaluation de l’influence des pharmaciens sur les résultats cliniques pour les patients. Ces données suggèrent que les pharmaciens des USI accordent la priorité aux activités cliniques destinées aux soins des patients les plus malades.
MOTS CLÉS: pharmacie clinique , consignation , résultats thérapeutiques , interventions , mortalité , durée du séjour
[Traduction par l’éditeur]
Although the concept of clinical pharmacy emerged in the late 1960s, there followed a period of at least 40 years during which pharmacy practice saw few changes relative to the progress that has taken place recently.1 Today, clinical pharmacy services are recognized for their significant contributions to optimal delivery of patient-centred care. In the intensive care unit (ICU), clinical pharmacists have established an indispensable role as part of the multidisciplinary health care team.2,3 Nonetheless, as health care and pharmacy services continue to change, it becomes crucial to evaluate if a given practice is achieving the primary goal of improving patient outcomes. Documentation and analytical methods are necessary to assess whether patients are benefiting from various clinical services, particularly in the ICU, where a greater level of care is often required. Such knowledge can guide decision-making for future practice and assist in the provision of optimal patient care.
Under the Health Professions Act of British Columbia,4 as well as similar requirements set by pharmacy associations, a pharmacist’s activities should be documented directly in the health care record. More specifically, the BC bylaw states that information on drug-related problems, recommendations, consultations, counselling, and clarifications should be part of the patient’s permanent health care record.4 There is no exception to this requirement in the ICU, where clinically important notes covering consultation requests, drug information responses, allergy history, drug therapy plans, and medication changes are to be documented directly in the patient’s chart to improve continuity of care and accountability. In general practice, drug-related problems are prioritized and documented according to their potential to cause significant decline in the patient’s condition. At the Royal Columbian Hospital (RCH) in New Westminster, British Columbia, all ICU patients are reviewed, but pharmacists generally provide documentation and devote more time to patients who are sicker or who have more acute conditions and those admitted more recently, because these patients are more likely to have active drug-related problems. Such documentation can be used for quality assurance and to satisfy Canadian accreditation requirements. Documenting directly in the health care record avoids duplication and makes the process more efficient.
In 1995, an innovative system was developed at RCH that enabled efficient documentation and measurement of pharmaceutical care activities. Together with the RCH Health Records Department, Gordon and others5 established a process that involved pharmacists writing a clinical pharmacy note (CPN) in the patient’s chart for each intervention, with each CPN being coded with the pharmacist’s identity, ward number, and intervention type. The documentation process used by Gordon and others5 was based on the abstracting system of the Health Records Department, which involves retrieval of specific information from patients’ charts. The information is entered into an electronic database; at the time of discharge, information in the database is used to generate a synopsis of the patient’s admission, in a format suitable for analysis. Gordon and others5 found that using the services of the Health Records Department not only helped to eliminate bias, but also resulted in more accurate reporting than would have been possible using information provided by the pharmacy department.
By examining how pharmacy services affect outcomes such as cost, mortality, and length of stay, Bond and others6 showed that as clinical pharmacist staffing levels increase, hospital mortality rate declines. However, few studies have evaluated pharmacist interventions in the ICU or examined the effects of clinical pharmacy services on “hard” clinical outcomes (such as mortality) directly reflecting tangible benefits to patients. The objective of the study reported here was to use the unique documentation system established at the RCH to evaluate the association between ICU patients’ severity of illness and clinical pharmacists’ interventions, and to compare mortality rates for patients with and without documented clinical pharmacy interventions. It was anticipated that the findings of this study would assist in the assignment of workload and the allocation of health care resources, as well as informing future research on clinical pharmacy services.
This observational study was conducted using inpatient data collected through the Health Records Department at RCH. Between 2004 and 2006, the adult ICU at this institution had 13 beds, and an additional bed was added in 2007. Although the ward was an adult ICU, adult-sized adolescents were occasionally admitted. The main study cohort comprised all patients with one or more ICU stays between January 1, 2004, and March 31, 2007. Patients were classified into 2 groups by the presence or absence of one or more CPNs recorded in the inpatient record during the ICU admission (“CPN group” and “no-CPN group”, respectively). In this study, the presence of a CPN was used as an indicator of clinical pharmacist interventions. The Health Records Department was able to use the ward location in the CPN code to specifically select CPNs that had been written in the ICU. CPN codes specifically captured clinical interventions; interventions not requiring cognitive assessment, such as automatic drug substitutions, would not be captured through these codes. Any notes written by pharmacy students were reviewed and coded along with the pharmacist preceptor’s name.
At the RCH, CPNs are used to document clinical activities such as medication therapy recommendations and clinical pharmacist interventions intended to optimize a patient’s drug therapy. More specifically, these activities and interventions may include provision of drug information, medication reconciliation at the time of admission, reporting of adverse drug reactions, clarifications of allergy history, and other clinical activities relevant to the identification and resolution of drug-related problems. Over the study period, all ICU patients, regardless of CPN status, were reviewed daily by ICU pharmacists during rounds. The hospital dispensary provided only minimal clinical support when ICU pharmacists were on site; instead, any potential drug-related issues identified in the dispensary would be brought to the attention of the ICU pharmacists for resolution. Although patients without CPNs would have received full rounds attendance in the ICU, those with CPNs would have received more in-depth review of their drug therapy by ICU pharmacists because of their medical status or the severity of their drug-related problems. For each patient, the Health Records Department assigned a complexity level on the basis of medical conditions present during the hospital stay, in accordance with the Canadian Institute for Health Information grouping methodology. The complexity or severity of illness ranged from 1 to 4, as defined in Appendix 1.
The variables in the data set included age, sex, complexity level, ICU length of stay (days), hospital length of stay (days), a dichotomous indicator for the outcome of death before discharge, and a dichotomous indicator for the presence of one or more CPNs in a patient’s record (see Appendix 1). For patients with multiple ICU admissions during a single hospital stay, the ICU length of stay was defined as the sum of ICU admissions. Characteristics of patients in the CPN and no-CPN groups were compared using descriptive statistics.
Because CPN was not a randomly assigned intervention in this study, multivariate logistic regression modelling was performed to control for potential confounding in the following evaluations.7 In the primary analysis, the association between complexity level (an ordinal explanatory variable) and CPN status (the outcome variable) was evaluated. The possible association between CPN status and mortality was also explored, first with the χ 2 test (2-sided α level 0.05) and then with logistic regression to adjust for potential confounding. The mortality analysis was also carried out in a matched analysis, in which the CPN and no-CPN groups were matched by complexity level, a predictor of mortality. The matched cohort included all patients in the CPN group and a subset of patients from the no-CPN group, randomly selected to match the proportion of patients in each complexity level in the CPN group. All data analyses were conducted using SPSS version 20 (Chicago, Illinois).
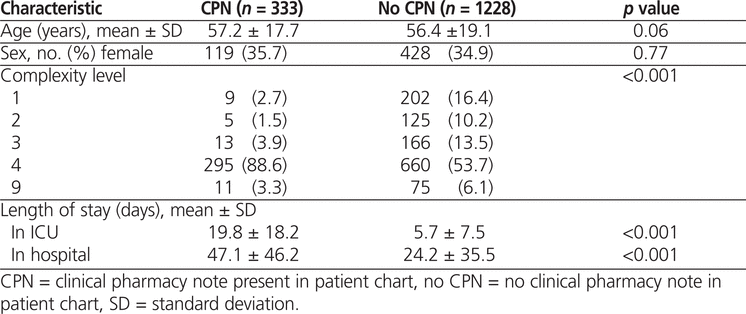
The main study cohort had a total of 1561 patients: 333 (21.3%) patients with CPNs and 1228 (78.7%) with no CPNs. Mean age and sex ratio did not differ significantly between the 2 groups (Table 1). However, the groups did differ in the distribution of complexity levels ( p < 0.001), with a greater proportion of patients in the CPN group than in the no-CPN group having complexity level 4, with potentially life-threatening illnesses (295/333 [88.6%] and 660/1228 [53.7%], respectively). In contrast, a smaller proportion of patients in the CPN group than in the no-CPN group had complexity level 1, the lowest level of complexity (9/333 [2.7%] and 202/1228 [16.4%], respectively). Both ICU and hospital lengths of stay were significantly longer for patients in the CPN group ( p < 0.001; Table 1). The mean difference in ICU length of stay was 14.1 days (95% confidence interval [CI] 12.8–15.4 days), and the mean difference in hospital length of stay was 22.9 days (95% CI 18.3–27.5 days).
Table 1.
Characteristics of Patients with and without Clinical Pharmacy Notes (CPNs)

There was a significant association between complexity level and CPN status. After adjustment for age and sex, the odds ratio for presence of a CPN among patients with complexity level 4 (relative to patients with lower complexity levels) was 8.20 (95% CI 5.44–12.38). The mortality rates in the 2 groups were not significantly different: 26.7% (89/333) among patients with CPNs and 27.9% (343/1228) among those with no CPNs ( p = 0.66) (Table 2). Univariate regression analysis showed that CPN status was not associated with mortality rate, and the crude odds ratio (OR) for CPN versus no CPN was 0.94 (95% CI 0.72–1.24). After adjustment for age, sex, complexity level, and length of stay in the ICU, the presence of a CPN remained not significantly associated with mortality (OR 0.86, 95% CI 0.62–1.18). Alternative models were constructed to examine the effect of different model inputs. In all iterations, CPN was not significantly associated with mortality. In the matched analysis, the cohort ( n = 1078) consisted of the 333 patients in the CPN group and 745 randomly selected patients from the no-CPN group. In the matched cohort, the mortality rates were 26.7% (89/333) in the CPN group and 30.3% (226/745) in the no-CPN group ( p = 0.23) (Table 2). The CPN variable was not significantly associated with mortality after adjustment for age, sex, complexity level, or ICU length of stay in the regression analysis for the matched cohort.
Using a unique documentation system established at the RCH, we described the characteristics of ICU patients who had clinical pharmacist interventions, evaluated the association between complexity level and CPN status, and assessed the possible impact of clinical pharmacy interventions on mortality rate over a 39-month period. Given the critical care setting of this study, the majority of patients in the main cohort had complexity level 4 (61.2%), with potentially life-threatening illnesses. Conversely, the majority of patients had no CPNs (78.7%), most likely as a result of limited clinical pharmacist staffing and high work volume. Notably, however, patients with complexity level 4 constituted 88.6% of the CPN group but only 53.7% of the no-CPN group. This finding appears to indicate that pharmacists selectively provided interventions to sicker patients in their routine clinical practice. Indeed, there was a statistically significant association between complexity level 4 and CPN status. In this study, the odds of a CPN being present in the chart among patients with complexity level 4 was approximately 8 times the odds among patients with lower complexity levels. This association persisted after adjustment for age and sex. The observed association between complexity and CPN status suggested that the clinical pharmacists prioritized services and documentation for sicker patients.
The CPN group had significantly longer lengths of stay in the ICU and in hospital than the no-CPN group. Since CPNs represent clinical pharmacy interventions and preceded discharge from both the ICU and the hospital, the length-of-stay measures were potential outcomes of the CPNs. However, patients with a longer length of stay may have had a greater likelihood of a CPN being recorded in the chart, relative to those with a shorter length of stay, because of an increased chance that new or additional drug-related problems would be identified during a longer stay. It is also possible that longer (eventual) lengths of stay reflected severe illness during the hospital stay (i.e., higher complexity level) which, as discussed above, might have prompted intervention by a pharmacist and hence the recording of a CPN. It is unlikely that pharmacists saw patients only after they had been admitted for a period of time (as opposed to soon after admission), as newly admitted patients and those with more acute conditions tend to have more active drug-related problems, which would prompt the involvement of a clinical pharmacist. Although the CPN group presumably received more in-depth review of their drug therapy because of their medical status or severity of drug-related problems, all patients were assessed by ICU pharmacists, irrespective of CPN status. Previous authors who evaluated length of stay, including Bjornson and others,8 have found that clinical pharmacy interventions (medication reconciliation, drug therapy plans, and discharge counselling) decreased the length of stay among patients in the intervention group. Similarly, Boyko and others9 showed that length of stay was reduced when clinical pharmacists were incorporated into general medical teams. This evidence, which contrasts with the findings of the current study, supports reductions in length of stay in association with clinical pharmacy interventions.
In this small cohort of 1561 patients, the presence of CPNs was not associated with a lower mortality rate. The effect of CPN on mortality was not statistically significant after adjustment for age, sex, level of complexity, and length of stay in the ICU. The effect was also not significant after the CPN and no-CPN groups were matched by complexity level, a predictor of mortality. These findings may have been limited by the small cohort size and the possible influence of unmeasured or inadequately controlled confounding, rather than a lack of impact. Furthermore, inaccuracies in data abstracting and miscoding were possible sources of error that could not be quantified. Although errors in data abstracting were assumed to be minimal, some clinical pharmacist interventions might not have been recorded if CPN codes were missing (e.g., for interventions performed by newly employed clinical pharmacists who had not yet been assigned a pharmacist code or in cases when clinical pharmacists neglected to document a CPN code in the chart). Such errors would have led to underreporting of interventions captured as CPNs and misclassification of patients. Moreover, the presence of a CPN might not have reflected the full scope of clinical pharmacist interventions in the ICU. Further research is needed to identify methods to accurately measure clinical pharmacist interventions in the ICU and to evaluate the effect of various types of intervention on clinical outcomes.
Many of the studies examining pharmacist interventions have had small sample sizes from one or only a few sites, short durations, and several uncontrolled confounding factors. The lack of consistency in methodology for evaluating interventions leads to difficulty in comparing study results. In turn, conflicting results in the literature make it difficult to draw conclusions on the impact of clinical pharmacy interventions. In their systematic review, Kaboli and others10 searched for literature regarding the effects of clinical pharmacy interventions on processes and outcomes. They found that in-hospital mortality rate had been analyzed in 8 of the 36 trials included in the review, and only one of these trials, involving an antimicrobial control program,11 demonstrated a statistically significant reduction in mortality; of the remaining 7 trials, 3 showed a lower mortality rate and 4 had higher mortality rates with pharmacy interventions, but these findings were not statistically significant.10 With such limited evidence and the tendency for trials to evaluate interventions in only one specific area of practice (e.g., renal monitoring or antimicrobial monitoring), determining where and to what extent patients benefit from clinical pharmacy interventions remains unclear.
Bond and Raehl12 recognized that large trials are needed to help develop clinical pharmacy practice and to reduce the potential bias associated with small, single-site studies. Using records from 1998, they analyzed data for 2 836 991 patients at 885 hospitals in the United States. In contrast to the results reported here, they found that half (7) of the 14 pharmacy services examined (drug-use evaluation, in-service education, management of adverse drug reactions, management of drug protocol, participation on cardiopulmonary resuscitation teams, participation on medical rounds, and admission drug histories) were associated with lower mortality rates.12 However, their analysis considered only overall hospital mortality rate. Studies examining mortality in the ICU remain scarce. MacLaren and Bond13 were the first to demonstrate lower mortality rate, shorter length of stay in the ICU, and lower costs when clinical pharmacists were directly involved in the care of ICU patients with thromboembolic or infarction-related events. They retrieved data from a large database of 141 079 patients,13 but, like the work of Bond and Raehl,12 their analysis was limited to correlating mortality rate with clinical pharmacy services. They did not account for numerous additional factors that may affect mortality rate, which makes it difficult to determine whether the presence of these services truly had an effect on overall mortality rate. Moreover, they evaluated outcomes only for a specific patient population within the ICU. In the current study, we used CPNs as recorded in administrative data as an indicator of pharmacists’ interventions and evaluated the clinical effect of CPNs at the individual patient level, under routine clinical practice conditions. This methodology can be adapted to clarify how pharmacists affect patient care in many clinical settings.
The current study had several limitations. First, the sample size was too small to detect a statistically significant difference in mortality. Although data for this patient population were collected beyond March 31, 2007, and inclusion of more recent data would have expanded the sample size, only 3 consecutive years of data were analyzed because the abstracting software used by the Health Records Department was changed in 2008. Future studies could analyze data from 2008 and beyond, to expand the sample size and study duration.
Furthermore, it is possible that not all interventions performed by clinical pharmacists were captured with CPNs. In practice, CPNs are often written to document drug kinetics, consultations, and clinically significant drug interactions. However, patients without documented CPNs might have received verbal recommendations or consultations from clinical pharmacists; in addition, regardless of CPN status, all patients were reviewed by ICU pharmacists. Basing the intervention tally only on documented interventions may have led to underestimation of the number of patients who received interventions and inclusion in the no-CPN group of patients for whom a pharmacist performed an intervention. As such, the results of this study may have been biased toward no effect, and the true impact of clinical pharmacy interventions may be greater than what was shown in this study.
The analyses were also restricted to a single study site, because the study was based on the unique documentation system previously established at this hospital by Gordon and others.5 This may limit the generalizability of these results to other ICUs.
The outcomes of some patients may have been misclassified, including those of patients discharged to other institutions or facilities. For example, if a patient was transferred to the ICU of another institution, the length of ICU stay recorded for this study would have been limited to data available in records at the study hospital and would not have included time in any other ICU.
Although the coding system used by the Health Records Department documents the intervention type and the total number of CPNs recorded for each patient, this study did not evaluate these variables. Other clinically relevant data, such as Acute Physiology and Chronic Health Evaluation (APACHE) scores and rates of ventilator-associated pneumonia, were also not included in the study. These parameters may be useful in future studies to identify specific patient groups that may require more clinical pharmacy services and clinical activities with greater impact, as well as to describe the types of interventions that pharmacists are performing. This information might assist in identifying patient populations or service areas where increased staffing or emphasis from clinical pharmacists could have a greater impact on patient care.
Documentation of pharmacy activities is an important component of clinical pharmacy practice. Together with the results of the previous study,5 this study has demonstrated that an established coding system for CPNs can be used to create a database suitable for analysis to evaluate the impact of clinical pharmacy services on various patient outcomes. Although this study showed no significant impact of CPNs on mortality rates in the ICU, future studies incorporating larger sample sizes, longer study periods, and multiple study sites might demonstrate significant differences in mortality rates and other outcomes. This process can be an effective tool for assisting with workload distribution, ensuring pharmacist accountability, and improving clinical practice. As clinical pharmacy continues to evolve and services continue to expand, this relatively new aspect of health care will require similar investigations to establish the role of clinical pharmacy practice and to maximize its positive impact on patient outcomes.
The documentation of clinical pharmacy activities and the ability to identify pharmacists’ interventions in electronic health records are essential for assessing pharmacists’ impact on patient outcomes. In this observational study, we found that pharmacists provided more in-depth care to patients with higher complexity levels, as evidenced by documentation of important information regarding drug-related problems. However, pharmacists’ interventions as documented in inpatient records were not associated with mortality rate. Further investigations are needed to more accurately measure the clinical impact of pharmacist services and to identify the types of interventions most beneficial to ICU patients.
1. Miller RR. History of clinical pharmacy and clinical pharmacology. J Clin Pharmacol. 1981;21(4):195–7.
2. Leape LL, Cullen DJ, Clapp MD, Burdick E, Demonaco HJ, Erickson JI, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282(3):267–70.
3. Kopp BJ, Mrsan M, Erstad BL, Duby JJ. Cost implications of and potential adverse events prevented by interventions of a critical care pharmacist. Am J Health Syst Pharm. 2007;64(23):2483–7.
4. Health Professions Act—bylaws. Schedule F, Part 2. Hospital pharmacy standards of practice. Vancouver (BC): College of Pharmacists of British Columbia; 2010 [cited 2012 Apr 27]. Available from: http://library.bcpharmacists.org/D-Legislation_Standards/D-2_Provincial_Legislation/5079-HPA_Bylaws_Hospital.pdf
5. Gordon W, Malyuk D, Taki J. Use of health-record abstracting to document pharmaceutical care activities. Can J Hosp Pharm. 2000;53(3):199–205.
6. Bond CA, Raehl CL, Pitterle ME, Franke T. Health care professional staffing, hospital characteristics, and hospital mortality rates. Pharmacotherapy. 1999;19(2):130–8.
7. Wunsch H, Linde-Zwirble WT, Angus DC. Methods to adjust for bias and confounding in critical care health services research involving observational data. J Crit Care. 2006;21(1):1–7.
8. Bjornson DC, Hiner WO Jr, Potyk RP, Nelson BA, Lombardo FA, Morton TA, et al. Effect of pharmacists on health care outcomes in hospitalized patients. Am J Hosp Pharm. 1993;50(9):1875–84.
9. Boyko WL, Yurkowski PJ, Ivey MF, Armitstead JA, Roberts BL. Pharmacist influence on economic and morbidity outcomes in a tertiary care teaching hospital. Am J Health Syst Pharm. 1997;54(14):1591–5.
10. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006; 166(9):955–64.
11. Gentry CA, Greenfield RA, Slater LN, Wack M, Huycke MM. Outcomes of an antimicrobial control program in a teaching hospital. Am J Health Syst Pharm. 2000;57(3):268–74.
12. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27(4):481–93.
13. MacLaren R, Bond CA. Effects of pharmacist participation in intensive care units on clinical and economic outcomes of critically ill patients with thromboembolic or infarction-related events. Pharmacotherapy. 2009;29(7):761–8.
Competing interests: None declared.
Canadian Journal of Hospital Pharmacy , VOLUME 66 , NUMBER 4 , July-August 2013