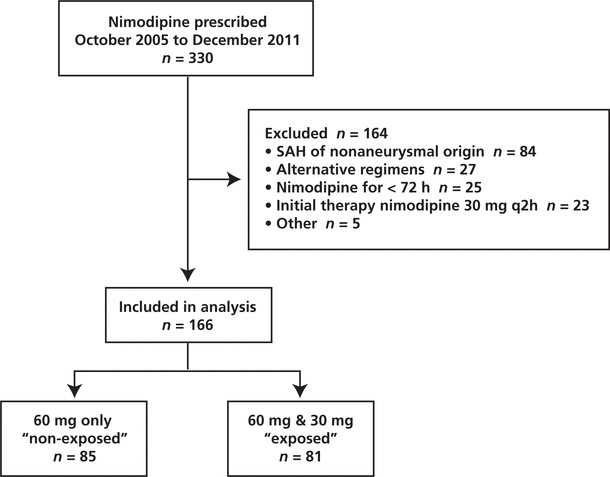
Figure 1. Flow of patients through the study. SAH = subarachnoid hemorrhage.
Meghan MacKenzie , Sean K Gorman , Steve Doucette , Robert GreenABSTRACT
Background:
Aneurysmal subarachnoid hemorrhage is a significant cause of death and disability. Nimodipine 60 mg administered enterally every 4 h improves neurologic outcomes in these patients. However, hypotension is an adverse effect of nimodipine and is believed to prompt clinicians to prescribe an unproven, nonstandard nimodipine dosing regimen.
Objectives:
The primary objective was to determine the prescribing incidence of a nonstandard nimodipine dosing regimen (30 mg every 2 h) after initial prescription of the standard dose (60 mg every 4 h). The secondary objective was to determine factors associated with this dosage change.
Methods:
This retrospective cohort study evaluated participants receiving nimodipine for aneurysmal subarachnoid hemorrhage at a tertiary care teaching hospital between October 2005 and December 2011. Univariate and multivariate regression analyses were performed to identify factors associated with dosage manipulation.
Results:
A total of 166 eligible patients were identified. For all of these patients, nimodipine 60 mg every 4 h was prescribed initially. Subsequently, 81 (49%) of the patients were switched to nimodipine 30 mg every 2 h, whereas 85 (51%) continued on the original dosage (nimodipine 60 mg every 4 h) for the duration of their treatment. Multivariate analysis revealed that occurrence of vasospasm (odds ratio [OR] 5.30, 95% confidence interval [CI] 2.08–13.47; p < 0.001) and exposure to vasopressor therapy (OR 3.29, 95% CI 1.27–8.50; p = 0.014) were associated with increased odds of receiving the nonstandard nimodipine regimen.
Conclusions:
Half of patients for whom nimodipine was prescribed for aneurysmal subarachnoid hemorrhage were exposed to an unproven regimen. Vasospasm and exposure to vasopressor therapy were associated with higher odds of receiving the nonstandard regimen. Further research is needed to evaluate whether nimodipine 30 mg every 2 h is efficacious and safe for patients in this population.
KEYWORDS: subarachnoid hemorrhage , nimodipine , dosage regimen
RÉSUMÉ
Contexte :
L’hémorragie sous-arachnoïdienne anévrismale représente une cause importante de mortalité et d’invalidité. L’administration par voie entérale de 60 mg de nimodipine toutes les 4 heures permet d’améliorer l’issue neurologique chez ces patients. Malheureusement, l’hypotension est un effet secondaire de la nimodipine et l’on croit que l’apparition de cet effet incite des cliniciens à prescrire un schéma posologique de nimodipine non standard et empirique.
Objectifs :
L’objectif principal visait à déterminer la fréquence de prescription d’un schéma posologique non standard de nimodipine (30 mg toutes les 2 heures) après une première prescription d’un schéma posologique standard (60 mg toutes les 4 heures). L’objectif second était de déterminer quels sont les facteurs associés à ce changement de schéma posologique.
Méthodes :
La présente étude de cohorte rétrospective observe les cas de participants qui ont reçu de la nimodipine, en raison d’une hémorragie sous-arachnoïdienne anévrismale, dans un hôpital universitaire de soins tertiaires entre octobre 2005 et décembre 2011. Des analyses de régression univariées et multivariées ont été menées afin d’identifier les facteurs motivant les changements au schéma posologique.
Résultats :
Au total, 166 patients admissibles ont été retenus. Tous ces patients se sont d’abord vu prescrire initialement 60 mg de nimodipine toutes les 4 heures. Par la suite, 81 d’entre eux (49 %) se sont vu prescrire 30 mg de nimodipine toutes les 2 heures, alors que 85 (51 %) continuaient de suivre le schéma posologique initial (60 mg toutes les 4 heures) pour la durée de leur traitement. Une analyse multivariée a révélé que les cas de vasospasmes (risque relatif approché [RRA] de 5,30, intervalle de confiance [IC] à 95% de 2,08–13,47; p < 0,001) et l’exposition à un traitement par vasopresseur (RRA de 3,29, IC à 95% de 1,27–8,50; p = 0.01) sont associés à une augmentation du risque pour le patient d’exposition au schéma posologique non standard.
Conclusions :
La moitié des patients qui se sont vu prescrire de la nimodipine en raison d’une hémorragie sous-arachnoïdienne anévrismale ont reçu un schéma posologique dont l’efficacité n’a pas été établie. La présence de vasospasme ainsi que l’administration d’un vasopresseur ont été liées à l’augmentation du risque pour le patient d’exposition au schéma posologique non standard. De plus amples recherches sont nécessaires pour évaluer l’efficacité et l’innocuité d’un schéma posologique de 30 mg de nimodipine toutes les 2 heures chez les patients de cette population.
MOTS CLÉS: hémorragie sous-arachnoïdienne , nimodipine , schéma posologique
[Traduction par l’éditeur]
Aneurysmal subarachnoid hemorrhage (aSAH), the extravasation of blood into the subarachnoid space following rupture of an intracranial aneurysm, is a relatively common event with a high rate of death and disability.1,2 After the initial rupture, the presence of blood in the subarachnoid space is directly toxic to the brain parenchyma, causing inflammatory responses, formation of free radicals, and dysregulation of vasoconstriction and vasodilatation.3 Complications resulting from these processes include delayed cerebral ischemia, cerebral vasospasm, and other medical and neurologic sequelae.4
Cerebral vasospasm is a leading cause of morbidity and mortality in patients after subarachnoid hemorrhage.5 Vasospasm is the narrowing of large-capacitance cerebral arteries at the base of the brain, resulting in reduced cerebral blood flow, secondary ischemia, and infarction.3 Nimodipine is a dihydropyridine calcium channel blocker that acts primarily by relaxing arterial smooth muscle, and its use has been associated with improved outcomes in patients with aSAH.6,7 Nimodipine exhibits cerebral vascular selectivity by preferentially dilating cerebral blood vessels to a greater degree than the peripheral and coronary vasculature.5,8 Clinical trials evaluating the efficacy of enteral nimodipine in patients with aSAH have used dosages ranging from 30 to 90 mg enterally every 4 h.9,10 The dosage presented in the Canadian Compendium of Pharmaceuticals and Specialties is 60 mg enterally every 4 h for 21 days after aSAH.6,11,12
Hemodynamic lability is independently associated with death or severe disability following aSAH.13 Avoidance of blood pressure fluctuations is a fundamental component of medical management in patients with aSAH.3,14,15 The authors of several review articles have recommended altering the dosage of nimodipine in patients experiencing hypotension (e.g., reducing the nimodipine dose to 30 mg and increasing the frequency of administration to every 2 h).16–19 However, this strategy has never been evaluated in terms of efficacy or impact on blood pressure.
Because of its effect as a calcium antagonist, nimodipine has the potential to cause systemic vasodilatation resulting in hypotension in certain individuals. However, according to FDA-approved labelling text for one proprietary nimodipine product, the overall estimated incidence of hypotension associated with enteral nimodipine is 5%.20 It has been suggested that adults with a history of hypertension seem to exhibit increased susceptibility to this effect, and the effect appears to be dose-related.12,20 The British Aneurysm and Nimodipine Trial reported the incidence of “hypotension” (without defining this term) as 0.4% (1/278) in patients receiving nimodipine 60 mg enterally every 4 h and 0.7% (2/276) in patients receiving placebo.21
Further evidence for the lack of a significant effect on blood pressure was outlined by Neil-Dwyer and others22 in a randomized trial evaluating the effect of nimodipine 60 mg enterally every 4 h versus placebo on daily average mean arterial pressure in 75 patients with various grades of aSAH. Mean arterial pressure was reduced by 5 mm Hg in the nimodipine group and by 1 mm Hg in the placebo group within the first 24 h of administration, but there was no difference in mean arterial pressure over the 21 days following this initial drop. The authors concluded that there was no alteration of blood pressure with nimodipine following aSAH.22 Petruk and others10 randomly assigned 154 patients with aSAH to nimodipine 90 mg enterally every 4 h or placebo. Mean systolic blood pressure (± standard deviation) was 140.6 ± 17.8 mm Hg in patients receiving nimodipine and 144.2 ± 19.6 mm Hg in those receiving placebo.10 Porchet and others23 attempted to determine the incidence of nimodipine-induced hypotension in patients after aSAH. Patients were given nimodipine 0.5 mg/h IV, with gradual titration to a maintenance dose of 2 mg/h and conversion to enteral administration when their condition improved; alternatively, patients considered to be in good clinical condition initially were started on nimodipine 60 mg enterally every 4 h. Of the 87 patients enrolled, 31 (36%) experienced mean arterial pressure of less than 75 mm Hg. In 26 of these 31 cases, the patients were receiving nimodipine by IV administration, whereas the other 5 were receiving the enteral regimen. These results suggest that lowering of blood pressure may be more significant at serum concentrations achieved with IV maintenance dosing.23
The information available to date in the literature, as summarized above, does not represent compelling evidence that nimodipine given enterally is associated with significant hypotension, yet according to our observations in practice, numerous clinicians employ the alternative dosing regimen of 30 mg enterally every 2 h, as suggested by experts.18
There is a lack of solid evidence that nimodipine is associated with significant decreases in blood pressure, the suggested alternative regimen of 30 mg enterally every 2 h lacks proven efficacy, and it is unknown if this alternative regimen has a different effect on blood pressure than the standard regimen. Therefore, the primary aim of this study was to determine, for the authors’ institution, the prescribing frequency of the nonstandard nimodipine dosing regimen (30 mg every 2 h) after initiation of the standard regimen (60 mg every 4 h) and to determine factors associated with use of the nonstandard regimen.
This retrospective cohort study involved patients who were admitted to QEII Health Sciences Centre, Halifax, Nova Scotia, from October 2005 to December 2011 for aSAH and who received nimodipine enterally. The hospital is a 1034-bed adult tertiary care referral and trauma centre and university-affiliated teaching hospital. The study protocol was internally reviewed, and approval was obtained from the Capital Health Research Ethics Board. Informed consent was not required by the institutional review board.
The pharmacy department’s drug-use evaluation database was used to identify adult patients (at least 17 years of age) who received nimodipine enterally. Patients were grouped according to their nimodipine regimen (standard or nonstandard, as described in the Introduction). The electronic health records of the identified patients were manually screened for a diagnosis of aSAH (as confirmed by computed tomography [CT]). All screening and data collection were performed by one investigator (M.M.). Patients who received at least one dose of nimodipine 60 mg enterally every 4 h and subsequently received 30 mg enterally every 2 h were compared with patients who received nimodipine 60 mg every 4 h for the entire duration of nimodipine therapy. Patients were excluded if they received nimodipine 30 mg every 2 h for the entire treatment duration, if they received other nimodipine dosage regimens, or if they received nimodipine for less than 72 h. Patients were also excluded if their subarachnoid hemorrhage had a nonaneurysmal cause, as documented in the medical record. The rationale for excluding patients who were started on a nonstandard regimen was the specific desire to understand what prompted a change in regimen. In addition, it was rationalized that application of this exclusion criterion would decrease the risk of indication bias, because patients initiated on a nonstandard regimen likely represent one or more different populations, which could potentially confound the results.
Data for patient characteristics and factors hypothesized to be associated with manipulation of the nimodipine regimen from 60 mg every 4 h (standard regimen) to 30 mg every 2 h (nonstandard regimen) were collected. The investigators included 2 critical care clinicians (a physician and a clinical pharmacy specialist [R.G. and S.K.G., respectively]) who frequently manage patients with aSAH. The choice of factors was based on a literature review and discussion within the investigative team, which led to a consensus on the following clinically relevant factors: age, sex, blood pressure (mean 24-h systolic blood pressure from 48 to 72 h after initiation of nimodipine), admission to the intensive care unit (ICU), occurrence of vasospasm at any time during nimodipine therapy, exposure to vasopressor therapy (norepinephrine or dopamine) at any time during nimodipine therapy, severe-grade aSAH (Fisher score 3 or 4 [for classifying appearance of subarachnoid hemorrhage on CT24; range 1 to 4] and/or Hunt and Hess score 4 or 5 [for classifying surgical risk25; range 1 to 5]), antibiotic exposure at any time during nimodipine therapy, and positive results on microbiology culture at any time during nimodipine therapy.16–19 The time frame for capturing blood pressure was chosen because nimodipine reaches steady state at about 48 h. Mean blood pressure over a 24-h period, rather than incident variations or extent of variations in blood pressure, was chosen primarily because the retrospective design of the study prevented investigation of alternative reasons for incident variations. In addition, clinicians may be less likely to change a dosage regimen on the basis of one isolated finding in a population that undergoes hourly blood pressure monitoring. Typical management of low blood pressure in this patient population may involve alternative strategies such as volume resuscitation; therefore, consistently low blood pressure, refractory to alternative interventions, was considered more meaningful because of its likelihood to prompt a change in therapy.18
Demographic and outcome data were reported with descriptive statistics. Categorical variables are summarized as frequencies, whereas continuous variables are shown as means and standard deviations or medians and interquartile ranges (IQRs), as appropriate. The proportions of outcome events were compared between nimodipine regimens with a χ 2 test statistic and are reported as odds ratios (ORs) with 95% confidence intervals (CIs). Other factors, as outlined above, were compared in a similar fashion. A multivariate logistic regression model was used to evaluate the factors associated with a change in nimodipine dosage, with adjustment for potentially relevant confounders. For a substantial proportion of the patients, no aSAH severity score was documented; therefore, a post hoc sensitivity analysis using the multivariate logistic regression model was performed to evaluate the factors associated with a change in the nimodipine dosage for patients with a documented aSAH severity score. Two-sided p values of 0.05 were considered statistically significant. Statistical analyses were performed using the SAS 9.1 software package (SAS Inc., Cary, North Carolina).
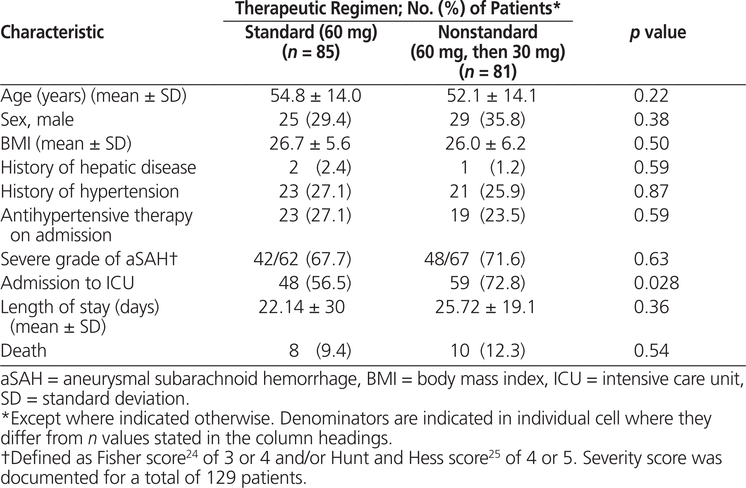
Between October 2005 and December 2011, 330 patients received nimodipine at the study institution, of whom 166 met the inclusion criteria for this study (Figure 1). Of these, 85 (51%) patients received the standard regimen for the entire duration of nimodipine therapy and the remaining 81 (49%) patients were switched to a nonstandard regimen of nimodipine (30 mg every 2 h) after having received at least one dose of the standard regimen (60 mg every 4 h). The median time to the switch in regimen was 2.58 days (IQR 1.33–7.25 days). The characteristics of the 2 groups were similar, except for a higher incidence of ICU admission among patients exposed to the nonstandard regimen (Table 1).
|
|
||
|
Figure 1. Flow of patients through the study. SAH = subarachnoid hemorrhage. |
||
Table 1.
Patient Characteristics
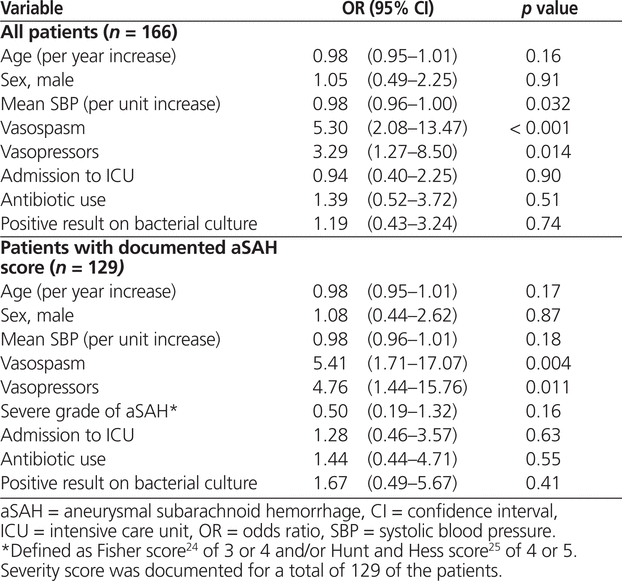
The unadjusted analysis revealed that occurrence of vasospasm (OR 6.95, 95% CI 2.34–20.66; p < 0.001) and exposure to vasopressors (OR 6.95, 95% CI 2.87–16.83; p < 0.001) were associated with manipulation of nimodipine dosage to the nonstandard regimen (Table 2). According to the multivariate analysis, the factors associated with nimodipine dosage manipulation remained the same: occurrence of vasospasm (OR 5.30, 95% CI 2.08–13.47; p < 0.001) and exposure to vasopressor therapy (OR 3.29, 95% CI 1.27–8.50; p = 0.014). However, an increase in mean systolic blood pressure was associated with significantly lower odds of manipulation to the nonstandard dosage regimen (OR 0.98, 95% CI 0.96–1.00; p = 0.032) (Table 3). For 37 (22%) of the patients included in the primary analysis (23 [27%] of those in the group with standard therapy and 14 [17%] of those in the group with nonstandard therapy), no aSAH severity score was documented. After exclusion of these 37 patients, vasospasm and vasopressor exposure remained significantly associated with dosage manipulation; however, increased systolic blood pressure was no longer associated with lower odds of exposure to the nonstandard nimodipine dosage (Table 3).
Table 2.
Univariate Analysis of Factors Associated with Changing Nimodipine 60 mg q4h (Standard Regimen) to Nimodipine 30 mg q2h (Nonstandard Regimen)
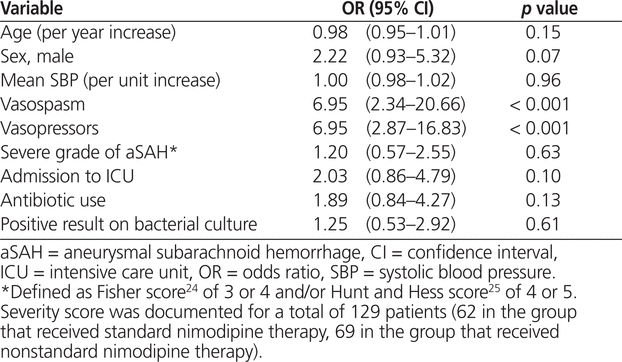
Table 3.
Multivariate Analysis Identifying Independent Predictors for Changing Nimodipine 60 mg q4h (Standard Regimen) to Nimodipine 30 mg q2h (Nonstandard Regimen)
Despite advances in the management of aSAH, nimodipine remains the pharmacotherapeutic intervention with the greatest benefit.2 Evidence-based best treatment of aSAH should include nimodipine at a dose that has been evaluated in randomized controlled trials. Nimodipine 30 mg every 2 h has not been evaluated in this way, and this dosage could therefore be considered to represent a drug-related problem. The current study was undertaken to determine the frequency of this potential drug-related problem at the authors’ institution and also to determine whether certain patient-related variables could explain why this nonstandard regimen was prescribed. Over the 6-year study period, about half of all patients who received nimodipine for aSAH at the study institution received the nonstandard regimen after initiation of therapy with the standard regimen. On the basis of our clinical experience and opinion-based recommendations in published reviews of nimodipine therapy for aSAH, we hypothesized that certain factors related to blood pressure were driving the dosage manipulation, including lower systolic blood pressure after reaching steady-state concentrations of nimodipine, need for vasopressor therapy, and occurrence of vasospasm. These 3 factors were indeed found to be associated with prescription of the nonstandard nimodipine regimen in this study.
The most recent clinical practice guidelines for aSAH recommend that the aneurysm be secured as early as possible to reduce the risk of rebleeding.2 After the aneurysm has been secured, the most significant complication is vasospasm, which can lead to delayed cerebral ischemia.2 In the current study, a significantly larger proportion of patients in the group who received the nonstandard regimen experienced vasospasm, and it is therefore plausible that clinicians initiated so-called “triple-H” therapy more often in these patients. Triple-H therapy involves ensuring that the patient has a hypervolemic state, is hemodiluted, and is hypertensive.2 The most common method to achieve hypertension is IV administration of vasopressors in combination with crystalloids. Perhaps clinicians altered the nimodipine regimen in these patients as an adjunct strategy to reduce the risk of hypotension. Conversely, clinicians may have been more comfortable continuing the standard dosage in patients with higher blood pressure, which might explain the lower odds of dosage manipulation in patients with higher blood pressure. However, after adjustment for the severity of aSAH, higher blood pressure was not associated with lower odds of receiving the nonstandard nimodipine regimen. The sample size was reduced by more than 20% in this sensitivity analysis, and it is therefore possible that analytical power was lost to detect an association between this factor and the type of nimodipine regimen.
This study had several limitations. The observational study design is subject to a number of biases and confounders. Selection bias may have been present, in that the different nimodipine dosage regimens may indicate the presence of 2 entirely different patient groups. Information bias may have been present, since completeness of health record documentation may have differed between the groups. For example, the frequency of missing aSAH severity scores was higher among patients exposed to the standard nimodipine regimen. This imbalance may reflect potential bias whereby participants for whom severity scores were not documented had lower severity of illness. It could be argued that these patients may have been more stable (e.g., fewer admissions to the ICU, decreased use of vasopressors, more stable blood pressure, fewer occurrences of vasospasm) and less likely to be switched from the standard regimen. Another reason for the missing entries may have been the study institution’s role as the major neurosurgical referral centre within the region, with many patients being transferred from other hospitals, where their initial CT and clinical exams would have been performed. It could be that only patients whose condition is stable can be transferred, and such patients may reflect a group with lower severity of aSAH. Multiple methods of severity scoring were allowed, and a nonvalidated system was used for defining severe aSAH according to Fisher and Hunt and Hess scoring. Specifically, a Fisher score of 3 or 4 and a Hunt and Hess score of 4 or 5 were considered severe, because patients with these scores have poor outcomes following aSAH.26–28 For many participants, only one of these severity scores was documented; therefore, severity of aSAH was analyzed in a dichotomous manner. The limitation of this analysis method is the assumption that the 2 scoring systems are concordant in terms of characterizing a patient’s risk for poor outcomes. For example, it is possible that a patient could have a high score on the Fisher score and a low score on the Hunt and Hess scale, but on the basis of the Fisher score, this patient would be coded as having severe-grade aSAH. The occurrence of vasospasm was captured from the physician’s progress notes and discharge summaries documented in the health record but was not necessarily radiographically confirmed; in the absence of radiographic confirmation, there might have been misclassification of vasospasm. Nonetheless, identical processes were used to capture information from the health records of patients in the 2 groups, which should have minimized the impact of these potential biases.
The ability to elucidate whether blood pressure lability was associated with manipulation of the nimodipine dosage regimen was hindered by the fact that half of the patients who were switched to the nonstandard regimen experienced the dosage change about 2.5 days after nimodipine initiation, and mean SBP was captured between 48 and 72 h following the first dose of nimodipine. It might have been possible to increase the number of patients in this analysis by including an additional 27 patients who were switched to other nonstandard regimens (e.g., 30 mg every 4 h). However, the basis of this study was the suggestion by experts that a specific unproven regimen (30 mg every 2 h) be used when patients experience a drop in systolic blood pressure, and it was thought that including patients on other nonstandard regimens would introduce additional bias. These 27 patients may represent a different patient population, and we cannot hypothesize why the various alternative regimens were employed. There was also a high suspicion of collinearity concerning blood pressure–related variables. For example, ICU admission, occurrence of vasospasm, and exposure to vasopressor therapy may be indicators of the same underlying clinical variable, given that vasopressor therapy is commonly used to treat vasospasm and a patient must be admitted to the ICU to receive vasopressors. In light of the possible associations among these variables, variance inflation factors were used to assess for possible collinearity. There was no statistical suggestion of collinearity, and the associations found were independent. This lack of statistical collinearity is clinically plausible, in that many of these patients could have received vasopressors for septic shock and could have been admitted to ICU for reasons unrelated to vasopressor therapy. In addition, the temporal relation between hypothesized variables and manipulation of the dosage regimen was not captured, which precluded determination of the direction of association between the variables explored and the outcome of interest (manipulation of nimodipine dosage). For example, the analyses revealed that the occurrence of vasospasm was independently associated with exposure to the nonstandard nimodipine dose. However, it is impossible to conclude whether the regimen was altered because of the onset or persistence of vasospasm or whether manipulation of the regimen led to vasospasm. The elapsed time from presentation to hospital to securement of the aneurysm was also not captured. This elapsed time has potential implications for blood pressure targets and possibly the nimodipine dosage regimen. For example, it is recommended that systolic blood pressure be kept below 160 mm Hg before securing the aneurysm, to reduce the risk of rebleeding; therefore, it is possible that prescribers who believed that nimodipine significantly affects hemodynamics continued the standard nimodipine regimen in these circumstances.29
The patient inclusion period for this study spanned 6 years, so there could have been a time-related treatment bias if nimodipine prescribing practices changed over time. In addition, the incidence of important outcomes such as delayed cerebral ischemia was not captured. The only outcome measure captured in this study that differed significantly between groups was ICU admissions, with an increased incidence of admissions in the group receiving the nonstandard nimodipine regimen. This difference may be explained by the different mix of clinicians caring for patients in the closed ICU setting, relative to the neurosurgical wards. It is possible that intensive care physicians have a perception of the hemodynamic effects of nimodipine that is systematically different from that of neurosurgeons. Another possible explanation for this difference was the significant association between vasopressor use and exposure to the nonstandard nimodipine dose. Policy at the study institution dictates that vasopressors can be administered only in critical care areas.
The strengths of this analysis included the systematic methods employed to identify the prescription frequency of the specified nonstandard regimen at a large, tertiary care academic hospital. The high prescribing frequency of the nonstandard nimodipine regimen observed in this study validates our speculation that this represents a potential drug-related problem. The internal validity of the analysis was strengthened by the method used to determine which factors to include in the regression analyses. Factors were identified by an intensive care physician and critical care pharmacy specialist on the basis of clinical plausibility, rather than mathematical modelling. Finally, to our knowledge, this is the first study to evaluate the frequency of prescribing a nonstandard nimodipine dosage regimen and to attempt to identify factors associated with its use. The results of this analysis have laid the groundwork for future research that should examine the efficacy and safety of the nonstandard regimen in this population.
Nimodipine dosage manipulation is an unproven strategy for managing aSAH and increases the opportunity for medication administration errors. Such errors have been associated with the complexity of medication orders.30–33 Dosing nimodipine every 2 h rather than every 4 h increases opportunities for medication administration–related errors, creating the potential for untoward effects in these patients. It should also be emphasized that nimodipine is a highly lipophilic compound with preferential action in the central nervous system and a low incidence of inducing hypotension. If the incidence of nimodipine-induced hypotension is truly 5%, then it appears that the perceived hypotension risk has been overestimated at the study institution.
A nonstandard nimodipine dosage regimen was prescribed at a frequency similar to that of standard dosage regimen. Blood pressure–related factors such as need for vasopressors and occurrence of vasospasm were associated with patients being switched to the nonstandard regimen. Higher blood pressure was protective against a change to the nonstandard regimen. Future efforts should focus on educating clinicians about the unknown efficacy and safety of the nonstandard regimen. Future study is needed to determine whether the nonstandard regimen is effective and safe for patients with aSAH.
1. Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354(4):387–96.

2. Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–37.

3. Rhoney DH, McAllen K, Liu-DeRyke X. Current and future treatment considerations in the management of aneurysmal subarachnoid hemorrhage. J Pharm Pract. 2010;23(5):408–24.
4. Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage. Neurosurg Clin North Am. 2010;21(2):325–38.
5. Liu-DeRyke X, Rhoney DH. Cerebral vasospasm after aneurysmal subarachnoid hemorrhage: an overview of pharmacologic management. Pharmacotherapy. 2006;26(2):182–203.

6. Dorhout Mees SM, Rinkel GJE, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007;(3):CD000277.
7. Rasmussen G, Bergholdt B, Dalh B, Sunde N, Cold G, Voldby B. Effect of nimodipine on cerebral blood flow and cerebrovascular reactivity after subarachnoid haemorrhage. Acta Neurol Scand. 1999;99(3):182–6.

8. Nimodipine monograph. In: Micromedex® healthcare series. Greenwood Village (CO): Thomson Micromedex; [cited 2011 Oct]. Available from: www.thomson-hc.com
9. Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Chou SN, et al. Cerebral arterial spasm—a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983;308(11):619–24.

10. Petruk KC, West M, Mohr G, Weir BK, Benoit BG, Gentili F, et al. Nimodipine treatment in poor-grade aneurysm patients. J Neurosurg. 1988;68(4):505–17.

11. Toyota BD. The efficacy of an abbreviated course of nimodipine patients with good-grade aneurismal subarachnoid hemorrhage. J Neurosurg. 1999;90(2):203–6.

12. Nimotop® [product monograph]. In: Compendium of pharmaceuticals and specialties [online version: e-CPS]. Ottawa (ON): Canadian Pharmacists Association; [cited 2011 Oct 29]. Available from: https://www.e-therapeutics.ca. Subscription required to access content.
13. Claassen J, Vu A, Kreiter KT, Kowalski RG, Du EY, Ostapkovich N, et al. Effect of acute physiologic derangements on outcome after subarachnoid hemorrhage. Crit Care Med. 2004;32(3):832–8.

14. Feigin VL, Findlay M. Advances in subarachnoid hemorrhage. Stroke. 2006;37(2):305–8.

15. Hinkel J, LeStrange DG. The pharmacologic treatment of vasospasm after subarachnoid hemorrhage: a case study. J Neurosci Nurs. 2003;35(6):332–5.
16. Alhashemi JA. Reduction of vasopressor requirement by hydrocortisone administration in a patient with cerebral vasospasm. Br J Anaesth. 2001; 86(1):138–41.

17. Rinkel G, Klijn C. Prevention and treatment of medical and neurological complications in patients with aneurysmal subarachnoid haemorrhage. Pract Neurol. 2009;9(4):195–209.

18. Rose JC, Mayer SA. Optimizing blood pressure in neurological emergencies. Neurocrit Care. 2004;1(3):287–99.
19. Rabinstein AA, Lanzino G, Wijdicks EF. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2010;9(5):504–19.

20. Nimotop® (nimodipine) capsules for oral use [FDA approved labelling text]. West Haven (CT): Bayer Pharmaceuticals; 2005 [cited 2014 May 22]. Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2006/018869s014lbl.pdf
21. Pickard JD, Murray GD, Illingworth R, Shaw MDM, Teasdale GM, Foy PM, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ. 1989;298(6674):636–42.


22. Neil-Dwyer G, Mee E, Dorrance D, Lowe D. Early intervention with nimodipine in subarachnoid haemorrhage. Eur Heart J. 1987;8 Suppl K:41–7.

23. Porchet F, Chioléro R, de Tribolet N. Hypotensive effect of nimodipine during treatment for aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien). 1995;137(1–2):62–69.
24. Fisher C, Kistler J, Davis J. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1–9.

25. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14–20.

26. Kosty T. Cerebral vasospasm after subarachnoid hemorrhage: an update. Crit Care Nurs. 2005;28(2):122–34.
27. Proust F, Hannequin D, Langlois O, Freger P, Creissard P. Causes of morbidity and mortality after ruptured aneurysm surgery in a series of 230 patients. Stroke. 1995;26(9):1553–7.

28. Kramer AH, Hehir M, Nathan B, Gress D, Dumont AS, Kassell NF, et al. A comparison of 3 radiographic scales for the prediction of delayed ischemia and prognosis following subarachnoid hemorrhage. J Neurosurg. 2008; 109(2):199–207.

29. Treggiari MM; participants in International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Hemodynamic management of subarachnoid hemorrhage. Neurocrit Care. 2011;15(2):329–35.

30. Hughes RG, Blegen M. Medication administration safety. In: Hughes RG, editor. Patient safety and quality: an evidence-based handbook for nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. p. 2–397 to 2–457.
31. Scott-Cawiezell J, Pepper GA, Madsen RW, Petroski G, Vogelsmeier A, Zellmer D. Nursing home error and level of staff credentials. Clin Nurs Res. 2007;16(1):72–8.

32. Stratton KM, Blegen MA, Pepper C, Vaughn T. Reporting of medication errors by pediatric nurses. J Pediatr Nurs. 2004;19(6):385–92.
33. Tang FI, Sheu SJ, Yu S, Wei IL, Chen CH. Nurses relate the contributing factors involved in medication errors. J Clin Nurs. 2007;16(3):447–57.

The authors would like to thank Heather Neville, BScPharm, MSc, Drug Utilization Pharmacist at Capital District Health Authority, for identifying patients from the drug-use evaluation database; Amanda Privett, Health Records employee at Capital District Health Authority, for compiling the charts for the identified patients; and Susan Bowles, PharmD, MSc, FCSHP, Clinical Coordinator and Residency Coordinator at Capital District Health Authority, for continued mentorship throughout the project, including statistical interpretation.
This study was supported by funding from the Division of Critical Care Research Committee, Dalhousie University, and by a Clinician Scientist Award to Robert Green from the Dalhousie University Faculty of Medicine.
Competing interests: None declared.
Canadian Journal of Hospital Pharmacy , VOLUME 67 , NUMBER 5 , September-October 2014