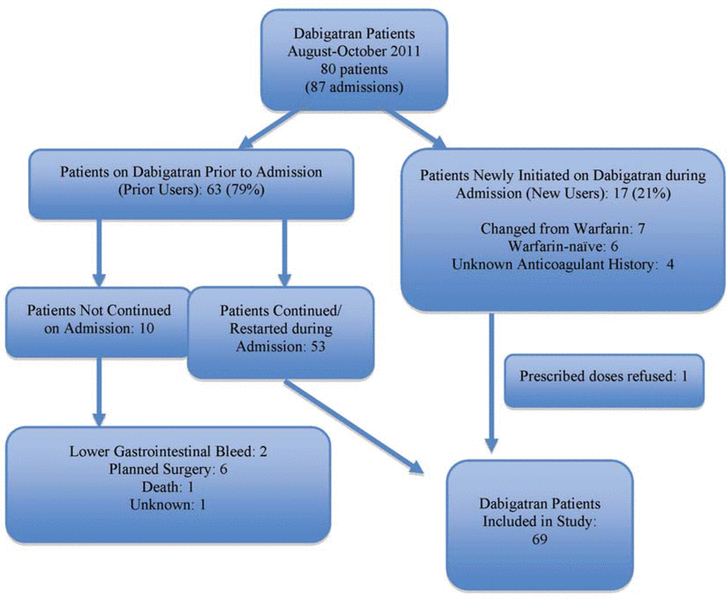
Figure 1. Patients identified for inclusion in a study to assess dabigatran therapy.
Aleesa A Carter, Kori Leblanc, Amita Woods, Donna LoweABSTRACT
Background:
The outpatient management of stroke prevention for patients with atrial fibrillation has recently been published and provides insight into the benefits and risks of the new direct-acting oral anti-coagulants. However, real-world use of these agents for hospital inpatients requires additional study.
Objective:
To determine prescribing patterns for dabigatran at 3 Canadian hospitals, specifically adherence with the hospitals’ prescribing restriction limiting dabigatran to patients with nonvalvular atrial fibrillation and creatinine clearance above 30 mL/min (primary outcome) and assessment of age-related prescribing, prescribing of medications with defined contraindications or potential for interaction when given concurrently with dabigatran, and use of risk stratification tools (secondary outcomes).
Methods:
A retrospective chart review of patients for whom dabigatran was prescribed from August to October 2011 was performed at 3 hospitals in Toronto, Ontario. Descriptive statistics were used for all outcomes assessed.
Results:
Overall, dabigatran was prescribed for 69 inpatients, of whom 16 (23%) were new users (dabigatran initiated during hospital admission) and 53 (77%) were prior users (dabigatran prescribed before admission to hospital). Fifty-eight patients (84%; 14 new users and 44 prior users) received dabigatran according to the hospitals’ prescribing restriction. For the remaining 11 patients, dabigatran therapy did not meet prescribing restrictions for use because of valvular disease or presence of prosthetic valve (10 patients [14% of the total sample]) and impaired renal function (1 patient [1%]). Among those whose dabigatran therapy met the prescribing restrictions for use, amiodarone and acetylsalicylic acid were the most common concurrently prescribed medications (17 patients [29%] and 14 patients [24%], respectively). Stroke and bleeding risk were documented for only 27 patients (47%) and 10 patients (17%), respectively.
Conclusion:
At the study hospitals, dabigatran was appropriately prescribed for the indication of nonvalvular atrial fibrillation in patients without renal impairment in most cases. However, greater consideration of cardiac history (including valvular disease and presence of prosthetic valves), drug interactions, and documentation of risks and benefits is warranted. These research findings highlight the importance of and opportunity for pharmacist review and involvement in assessment and selection of patients with indications for anticoagulant therapy, particularly when agents are new to the market.
KEYWORDS: arrhythmia, novel oral anticoagulants, drug use evaluation, hospital prescribing
RÉSUMÉ
Contexte :
La publication récente sur la prévention des accidents vasculaires cérébraux (AVC) chez les patients externes atteints de fibrillation auriculaire permet de mieux comprendre les avantages et les risques des nouveaux anticoagulants oraux directs. Cependant, il est nécessaire de faire de plus amples études sur l’utilisation de ces agents en situation réelle chez les patients hospitalisés.
Objectifs :
Déterminer les habitudes de prescription de dabigatran dans trois hôpitaux canadiens, particulièrement en ce qui a trait au respect des restrictions de prescription en vigueur dans les hôpitaux qui limitent le dabigatran aux patients souffrants de fibrillation auriculaire non valvulaire et présentant une clairance de la créatinine supérieure à 30 mL/min (principal paramètre d’évaluation) et à l’évaluation de la prescription en fonction de l’âge du patient, de la prescription de médicaments avec des contre-indications précises ou un potentiel d’interactions médicamenteuses lorsqu’ils sont administrés en concomitance avec du dabigatran et de l’emploi d’outils de stratification du risque (paramètres d’évaluation secondaires).
Méthodes :
Une analyse rétrospective des dossiers médicaux des patients à qui on avait prescrit du dabigatran entre août et octobre 2011 a été menée dans trois centres hospitaliers de Toronto en Ontario. Des statistiques descriptives ont été employées pour tous les paramètres analysés.
Résultats :
Dans l’ensemble, on a prescrit du dabigatran à 69 patients hospitalisés. Parmi eux, 16 (23 %) n’en avaient jamais reçu (traitement amorcé pendant l’hospitalisation) et 53 (77 %) en avaient déjà reçu (dabigatran prescrit avant l’hospitalisation). Cinquante-huit patients (84 %; 14 n’en ayant jamais reçu et 44 en ayant déjà reçu) ont reçu du dabigatran selon les restrictions de prescription en vigueur dans les hôpitaux. Pour les 11 patients restants, le traitement par dabigatran ne répondait pas aux restrictions d’utilisation pour cause de valvulopathie ou de présence d’une prothèse valvulaire (10 patients [14 % de l’échantillon total]) ou d’insuffisance rénale (1 patient [1 %]). Au sein du groupe de patients pour lesquels les restrictions d’utilisation ont été respectées, l’amiodarone et l’acide acétylsalicylique étaient les médicaments les plus souvent coprescrits (respectivement, 17 patients [29 %] et 14 patients [24 %]). Le risque d’AVC et d’hémorragie n’était consigné respectivement que pour 27 patients (47 %) et 10 patients (17 %).
Conclusion :
Dans les hôpitaux de l’étude, le dabigatran était habituellement prescrit de façon appropriée pour l’indication de fibrillation auriculaire non valvulaire chez des patients ne présentant pas d’insuffisance rénale. Cependant, il est justifié de tenir davantage compte des antécédents cardiaques (notamment les valvulopathies et la présence de prothèses valvulaire), des interactions médicamenteuses ainsi que de la consignation des risques et des avantages. Ces données mettent en relief l’importance et la possibilité de la participation du pharmacien à l’évaluation et à la sélection des patients ayant des indications pour un traitement par anticoagulant ainsi que de son analyse de ces cas, particulièrement lorsque les médicaments sont nouveaux sur le marché.
MOTS CLÉS: arythmie, nouveaux anticoagulants oraux, évaluation de l’utilisation des médicaments, prescription en milieu hospitalier
The emergence of newer oral anticoagulants for patients with atrial fibrillation has provided clinicians with an armamentarium of potential options for stroke prevention in the past several years.1–4 However, real-world use of these agents has remained a source of debate, and postmarketing reports (including reports of serious bleeding events and risks associated with use in patients with valvular atrial fibrillation or reduced renal function) have highlighted the need for further assessment in clinical practice.5–9 Dabigatran, the first competitive and reversible thrombin inhibitor approved in North America, in October 2010, has been evaluated in several outpatient registries of patients with atrial fibrillation in both Canada and the United States.10–12 However, its use in hospital inpatients warrants additional study.
Dabigatran (110 mg and 150 mg formulations) was added to the formulary of the study hospitals in June 2011. This drug was restricted to the prevention of stroke in patients with nonvalvular atrial fibrillation and creatinine clearance (CrCl) greater than 30 mL/min. The current study examined the appropriateness of dabigatran use relative to these criteria, along with compliance with Canadian labelling, which recommends a regimen of 110 mg twice daily for patients over 80 years of age and those over 75 years of age with increased risk of bleeding.13 The use of concomitant interacting or contraindicated medications was also characterized, as was documentation of tools used to assess risk of stroke and bleeding, consistent with recent guideline recommendations.14
This study was a 3-month retrospective chart review of all patients for whom dabigatran was prescribed between August and October 2011 at 3 tertiary care hospitals in Toronto, Ontario (about 700 beds total). During the period under review, dabigatran was the only novel oral anticoagulant available on the hospital network’s formulary and was not yet covered by the provincial formulary for outpatients. The University Health Network Research Ethics Board approved the study.
Patients were identified from the hospital network’s pharmacy computer system, which contains medication orders for all inpatients. Each patient’s individual medication administration record was reviewed, and patients were included in the analysis if dabigatran had been ordered during their inpatient stay and at least one dose of dabigatran had been administered. For patients with multiple admissions during the study period, only the first admission was included in the analysis.
Patients were categorized as prior users (dabigatran prescribed before admission to hospital) or new users (dabigatran newly initiated during the admission). Multiple sources were reviewed to categorize each patient, including the patient’s medication records in the pharmacy computer system and the electronic patient record (which is also a computerized prescriber order-entry system), past medication history and clinical notes in the patient’s paper chart, and the hospital network’s electronic best possible medication history database. The documented indication for use of dabigatran was obtained from a mandatory field in the electronic patient record, which required the prescriber to specify the indication for use before the order could be processed, as well as the written clinical progress notes in the patient’s chart. The approved indication of nonvalvular atrial fibrillation was further confirmed using the electronic patient record and the patient’s chart for either written or echocardiographic confirmation of the absence of moderate-to-severe mitral and/or aortic stenosis. An additional analysis was performed to exclude patients with prosthetic valves, given data that became available after initial prescribing indications were approved for use at the study hospitals.7,8 Renal function was based on the Modification of Diet in Renal Disease (MDRD) calculation available to the prescribing physician on the electronic order system and assessed for the duration of the patient’s hospital stay.15,16 If the calculated renal function (i.e., CrCl) decreased below 30 mL/min during the hospital stay, use of dabigatran was classified as inappropriate. Data were collected using a standardized data collection form and were entered into Microsoft Excel, version 12.3.2 (Microsoft Corporation, Redmond, Washington), for analysis.
The primary outcome measure was the proportion of patients meeting both aspects of the hospital network’s approved prescribing indication for dabigatran use: (1) nonvalvular atrial fibrillation (with modified criteria to exclude patients with prosthetic valves) and (2) CrCl greater than 30 mL/min. Secondary outcome measures were (1) the proportion of patients for whom an appropriate dose of dabigatran was prescribed, based on the patient’s age and prespecified risk factors for bleeding (history of gastrointestinal bleeding, intracranial hemorrhage, or known bleeding disorder), (2) the proportion of patients with prescriptions for concomitant contraindicated medications (specifically, P-glycoprotein inducers [protease inhibitors, dexamethasone], anticoagulants [unfractionated heparin, low-molecular-weight heparin, warfarin], azole antifungals) and the proportion of patients with concomitant medications requiring increased monitoring (specifically, P-glycoprotein inhibitors [amiodarone, dronaderone, verapamil, quinidine, tacrolimus], antiplatelet agents [acetylsalicylic acid, clopidogrel], anti-inflammatory agents); and (3) the frequency of documentation of stroke and bleeding risk scores during the patient’s hospital stay.
The investigators calculated both the CHADS2 and the CHA2DS2-VASc risk scores for all patients, except those for whom these risk stratification schemes were not applicable, such as patients with mitral stenosis.17,18 The CHADS2 risk score is a measure of stroke risk in which presence of congestive heart failure, hypertension, age 75 years or older, and diabetes mellitus are each assigned 1 point, and history of previous stroke or transient ischemic attack is assigned 2 points. The CHA2DS2-VASc score is another stroke risk tool, specifically aimed at stratifying risk for lower-risk patients by further assigning 1 point for each of female sex, age 65–74 years, and vascular disease (prior myocardial infarction, peripheral arterial disease, or aortic plaque) and 2 points for age over 75 years or history of thromboembolism. They also calculated the HAS-BLED risk score (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio (INR), elderly, drugs/alcohol concomitantly) to allow stratification of each patient’s bleeding risk, according to availability of the laboratory results required for this calculation; if such results were not available, the para meter was assumed to be normal.19,20
Descriptive statistics (proportions) were used to summarize all outcome measures. Outcome measures were examined for the overall group and for the subgroups of new and prior users.
Dabigatran was prescribed for a total of 80 patients (87 admissions) over the 3-month study period. Of these, 63 patients (79%) were prior users and 17 patients (21%) were new users; however, one patient in the latter subgroup refused the prescribed treatment and was excluded. Among the 63 prior users, 53 (84%) continued taking dabigatran during their hospital stay or, if the drug was initially held upon admission, had the drug restarted. The 10 prior users whose dabigatran therapy was not continued during the hospital stay were excluded from the assessment of in-hospital use (see Figure 1). Overall, for the 69 patients who were included in this study, mean age was 68 years (standard deviation 15 years), two-thirds (46 [67%]) were men, and two-thirds (46 [67%]) had a planned admission for various cardiovascular procedures (Table 1).
|
|
||
|
Figure 1. Patients identified for inclusion in a study to assess dabigatran therapy. |
||
Table 1. Characteristics of Patients with Prescriptions for Dabigatran Therapy (part 1 of 2)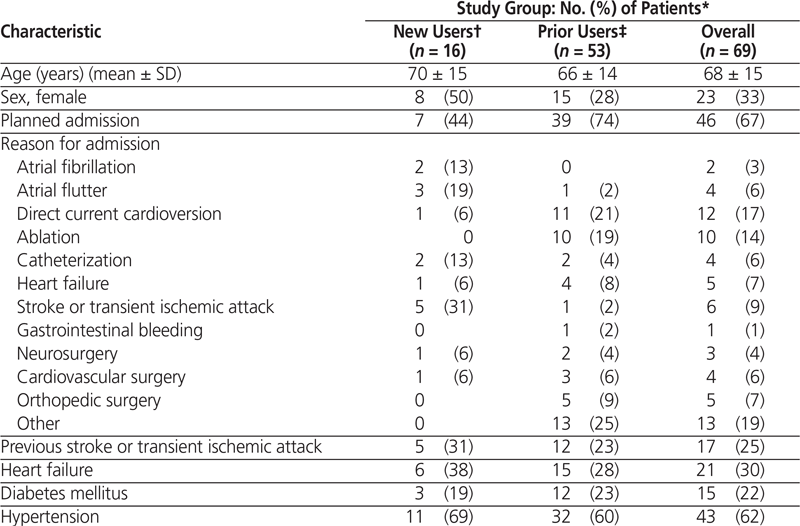
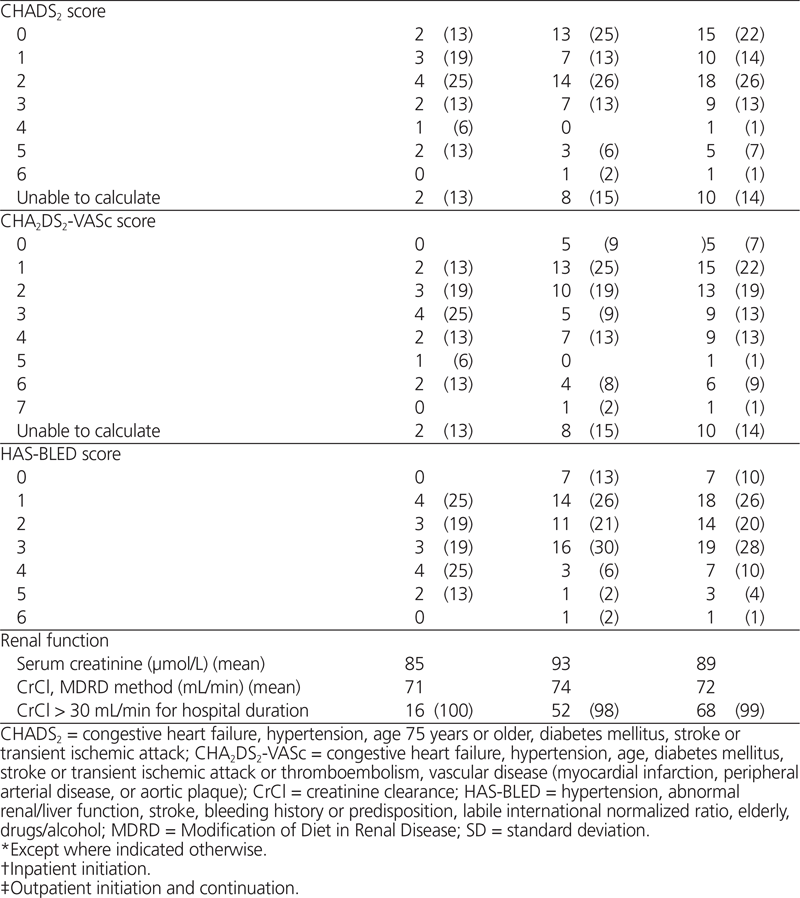
Among the 69 patients, 60 (87%) had nonvalvular atrial fibrillation and CrCl greater than 30 mL/min (Table 2), and therefore met the hospital network’s initial prescribing restrictions for use. When patients with prosthetic valves were excluded, appropriateness of use decreased to 58 patients (84%). Among these 58 patients, the appropriate dose, based on age and risk factors for bleeding, was prescribed for 55 patients (95%). Unfortunately, 4 patients (7%) received dabigatran concurrently with another anticoagulant (concurrent deep vein thrombosis prophylaxis for 3 patients and full anticoagulant treatment dosing for 1 patient; see Table 3). Overall documentation rates for stroke risk and bleeding risk were low (27 patients [47%] and 10 patients [17%], respectively). Interestingly, the CHADS2 score was calculated for only 4 (44%) of the 9 patients whose orders were written on a preprinted cardiovascular procedure form, with a dedicated space for calculation of the patient’s CHADS2 score in the medical record.
Table 2. Outcomes for Primary and Secondary Objectives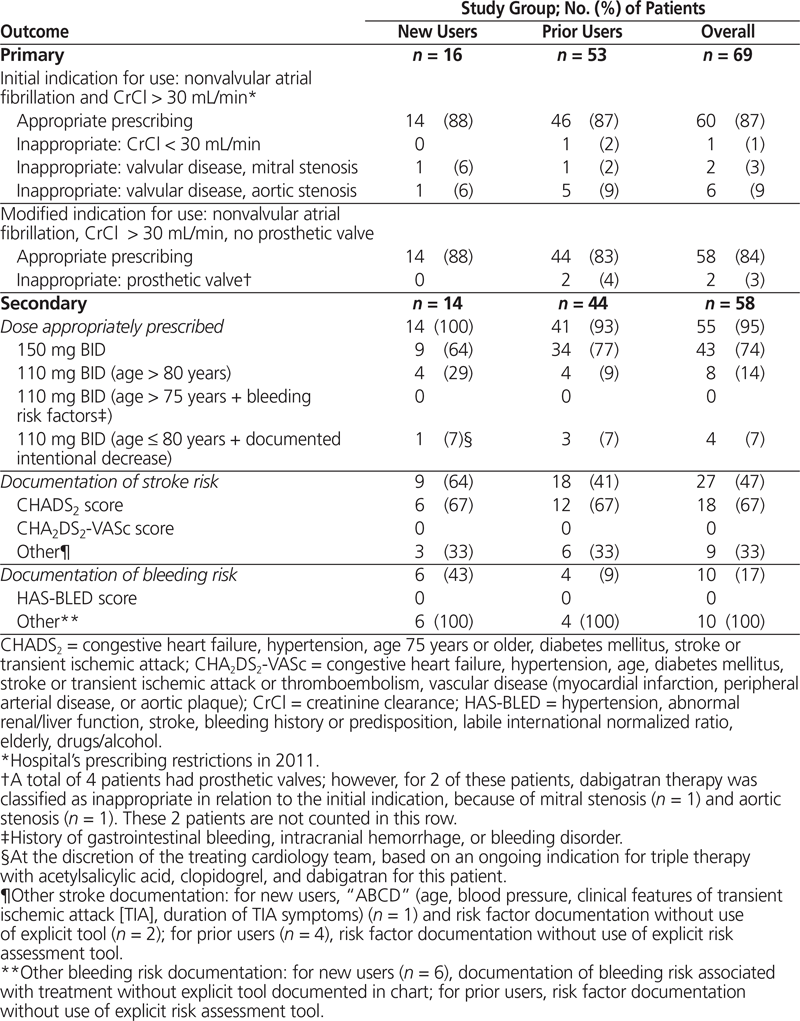
Table 3. Medications Prescribed Concurrently with Dabigatran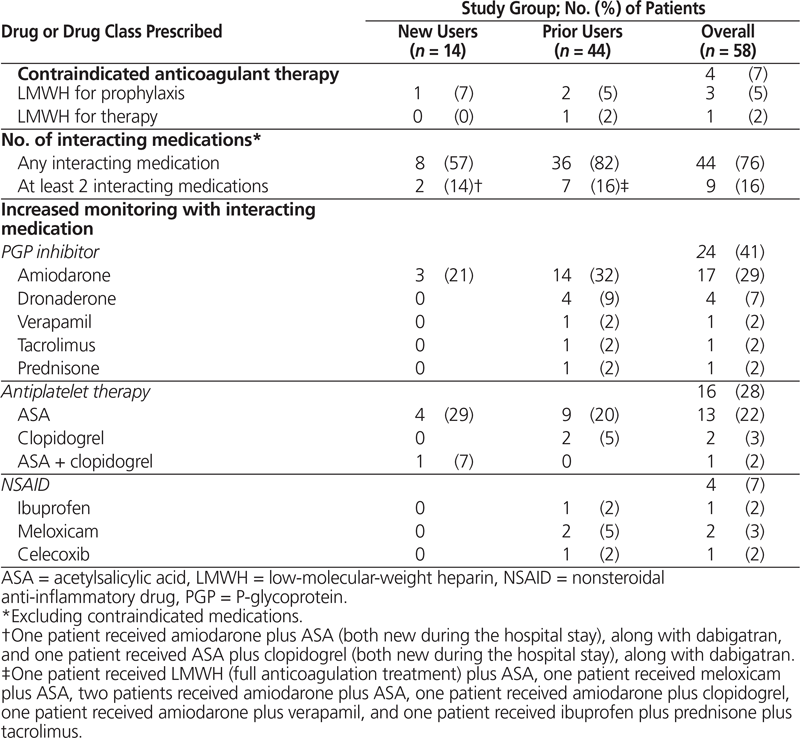
Among the 16 new users identified over the study period, dabigatran was appropriate for 14 patients (88%), according to the hospital’s prescribing restrictions (Table 2). Three of these patients (21%) were switched from warfarin because of subtherapeutic international normalized ratios at the time of admission. All 3 of these patients presented to hospital secondary to cerebrovascular events (2 patients with stroke, 1 patient with transient ischemic attack). The 2 patients whose prescribed dabigatran therapy did not meet the prescribing restriction for nonvalvular atrial fibrillation had a known history of severe mitral stenosis (with documented history of rheumatic heart disease) and a known history of severe aortic stenosis, respectively. None of the new users had renal impairment at initiation of therapy or at any time during their admission.
For all of the 14 new users, the appropriate dose was prescribed: 9 patients (64%) were to receive 150 mg twice daily, 4 patients (29%) patients were to receive 110 mg twice daily on the basis of their age, and 1 patient, despite being younger than 80 years of age, was to receive 110 mg twice daily after percutaneous coronary intervention during the hospital admission. For this latter patient, the intentional decision to prescribe a lower dose than that recommended for the patient’s age was documented in the patient’s medical record by the prescribing cardiology team because of an ongoing indication for dual antiplatelet therapy and dabigatran, and on this basis was categorized as appropriate.
Among the 14 new users, 8 (57%) had concurrent prescriptions for dabigatran and at least one interacting medication (Table 3). Of greatest concern was one patient who was receiving a low-molecular-weight heparin for deep vein thrombosis prophylaxis during treatment with dabigatran. Of agents with the potential to increase bleeding risk, concurrent antiplatelet therapy with acetylsalicylic acid (ASA) was most commonly prescribed (5 patients [36%]). ASA was newly prescribed for only one patient, whereas the remaining 4 patients (29%) were already taking ASA at the time of admission and the antiplatelet regimen was continued during the hospital stay and at discharge. Two patients who were taking ASA were also receiving a second interacting medication: one was the patient taking ASA and clopidogrel following percutaneous coronary intervention (as previously described), and the second patient was receiving amiodarone.
Stroke assessment was documented for 9 (64%) of the new users with appropriate indications for use of dabigatran. The CHADS2 score was used for 6 (67%) of these cases. Bleeding risk, without formal use of a risk assessment tool such as the HAS-BLED score, was documented for 6 (43%) new users.
For the 53 prior users who were continued on dabigatran therapy during their admission, 46 (87%) met the hospital’s initial prescribing restrictions (i.e., nonvalvular atrial fibrillation and CrCl over 30 mL/min). Also among the 53 prior users were 4 patients (8%) with a known history of a prosthetic valve; however, 2 of these patients were already identified as receiving dabigatran therapy inappropriately, because they had mitral stenosis (1 patient) and aortic stenosis (1 patient). According to the modified prescribing restriction (based on data that became available after initial hospital formulary approval), the remaining 2 patients with prosthetic valves were deemed to be receiving dabigatran inappropriately, which left 44 patients (83%) with appropriate indications for use. Among prior users, 1 patient (2%) did not meet prescribing criteria because of impaired renal function (CrCl 16 mL/min).
Among the 44 prior users meeting the hospital’s modified criteria for use, the dose of dabigatran ordered was appropriate for 41 patients (93%), on the basis of age and other bleeding risk factors. The remaining 3 patients were older than 80 years of age and were inappropriately continued on their home regimens of 150 mg twice daily. Additional anticoagulant therapy was concurrently prescribed for 3 patients: 2 receiving prophylactic therapy and 1 receiving full treatment dosing of a low-molecular-weight heparin. P-glycoprotein inhibitors were the medications most commonly prescribed concurrently with dabigatran (19 patients [43%]). Overall, stroke risk assessment was documented in the charts of 18 patients (41%), 12 (67%) of whom were assessed with the CHADS2 scoring tool. Bleeding risk was documented for 4 (9%) of the prior users, without use of any specific risk stratification tool such as HAS-BLED.
During the study period, prescribing of dabigatran met the hospital’s prescribing restriction for the majority of patients, according to the original criteria for formulary approval. The use of dabigatran for patients with valvular disease, as observed in this chart review, predated early communication of updated contraindications for use in patients with valvular disease or heart valve replacement.7 The RE-ALIGN study, which evaluated the use of dabigatran in patients with mechanical heart valves, demonstrated an increased risk of thromboembolic complications (stroke 5% versus 0%, myocardial infarction 2% versus 0%, and valve thrombosis 3% versus 0%) and bleeding complications (27% versus 12%; hazard ratio 2.45, 95% confidence interval 1.23–4.86) relative to warfarin.8 Given the retrospective nature of the current study, it was possible to review the appropriateness of dabigatran therapy in relation to this new evidence. The current study included 4 patients with prosthetic valves who were receiving dabigatran. This finding indicates the importance of re-evaluating appropriateness whenever new evidence of either benefit or harm becomes available, to further define the subset of patients who should or should not continue or initiate treatment.
This chart review also provided insight into areas of clinical practice where pharmacists can have a direct impact, including the assessment of dabigatran use according to renal function, age, and concomitant interacting medications. According to current prescribing recommendations, dose adjustments based on age were being instituted at the study hospitals for the majority of patients; however, dose adjustments accounting for the significance of interacting medications continue to be left to prescribers’ clinical judgment. For example, in this study, for one new user receiving dabigatran, ASA, and clopidogrel, an intentionally reduced dose of dabigatran was prescribed to minimize bleeding risk, whereas a prior user who was receiving dabigatran, prednisone, tacrolimus, and verapamil continued to receive dabigatran 150 mg twice daily. These examples highlight differing approaches to drug–drug interactions in clinical practice. As more evidence becomes available concerning the potential bleeding risk associated with such concurrent treatments,21 pharmacists are well positioned to discuss these interactions with prescribers in order to mitigate patient risk and ensure appropriate monitoring and patient counselling.
The results of this study also highlight the opportunity for pharmacists to engage in documentation of risk assessment. Despite current recommendations for documentation of stroke risk,14 this study demonstrated a significant gap between guideline recommendations and current practice for patients with atrial fibrillation. Pharmacists are well positioned to bridge this gap, particularly as therapeutic alternatives for stroke prevention in atrial fibrillation continue to undergo review and development.
A number of limitations in this study deserve mention. First, as with any chart review, the analysis was limited to information included in the patients’ charts or otherwise documented by the health care team. For example, for calculation of a patient’s HAS-BLED score, it was assumed that the patient had no history of labile INR if no INR was ordered at the time of admission and no mention was made of prior history of liver dysfunction; as a result, the calculated scores may have underestimated the true risk of bleeding. However, the same information would have been available to prescribers at the time dabigatran was prescribed. Second, the MDRD method was used as a measure of CrCl greater than 30 mL/min, rather than the traditional Cockcroft–Gault equation. However, renal function was followed throughout each patient’s hospital admission, and the MDRD method was used as a practical way of once again reflecting information that would have been available to physicians at the time of prescribing. Third, it is acknowledged that the list of interacting medications considered here was not exhaustive and that other interactions may be of importance (e.g., interactions with rifampin or anticonvulsants). Finally, in defining appropriateness, it is recognized that anticoagulation selection must take into account patient-specific preferences, which may not always be documented in the chart.
For the majority of inpatients in this study, dabigatran was appropriately prescribed, in accordance with restrictions for use in patients with nonvalvular atrial fibrillation and CrCl greater than 30 mL/min. Pharmacists are well positioned to assess and intervene upon the appropriateness of dabigatran use for patients with nonvalvular atrial fibrillation, according to usual prescribing recommendations and with consideration of patient age and renal function, as well as valvular status and bleeding risk factors, including concurrent interacting medications. Systems for improving the documentation of risk stratification are required to improve adherence with recent guideline recommendations, particularly given the armamentarium of agents available on the Canadian market.
1. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al.; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
2. Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L; Randomized Evaluation of Long-Term Anticoagulation Therapy Investigators. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363(19):1875–6.
3. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al.; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17.
4. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al.; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91.
5. Southworth MR, Reichman, ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med. 2013;368(14):1272–4.
6. FDA drug safety communication: safety review of post-market reports of serious bleeding events with the anticoagulant Pradaxa (dabigatran etexilate mesylate). Silver Spring (MD): US Food and Drug Administration; 2011 [cited 2015 Apr 1]. Available from: www.fda.gov/Drugs/DrugSafety/ucm282724.htm
7. Pradax (dabigatran etexilate) - updated labelling regarding kidney function assessment and use in patients with certain types of heart valve disease or artificial heart valves - for health professionals. Ottawa (ON): Health Canada; 2012 [cited 2015 Apr 1]. Available from: www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2012/15855a-eng.php
8. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al.; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–14.
9. Moore TJ, Cohen MR, Mattison DR. Dabigatran, bleeding, and the regulators. BMJ. 2014;349:g4517.
10. Xu Y, Holbrook AM, Simpson CS, Dowlatchahi D, Johnson AP. Prescribing patterns of novel oral anticoagulants following regulatory approval for atrial fibrillation in Ontario, Canada: a population-based descriptive analysis. CMAJ Open. 2013;1(3):E115–9.
11. Steinberg BA, Holmes DN, Piccini JP, Ansell J, Change P, Fonarow GC, et al.; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators and Patients. Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000535.

12. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131(2):157–64.
13. Pradaxa (dabigatran) [product monograph]. Burlington (ON): Boehringer Ingelheim Canada Ltd; 2011 [cited 2011 Dec 27]. Available from: www.boehringeringelheim.ca/content/dam/internet/opu/ca_EN/documents/humanhealth/product_monograph/PradaxaPMEN0513.pdf
14. Estes NAM 3rd, Halperin JL, Calkins H, Ezekowitz MD, Gitman P, Go AS, et al. ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Performance Measures for Atrial Fibrillation). Circulation. 2008;117(8):1101–20.
15. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
16. Levey AS, Coresh J, Green T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. Erratum in: Ann Intern Med. 2008; 149(7):519.
17. Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Boode BS, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110(16):2287–92.
18. Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D’Agostino RB, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290(8):1049–56.
19. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010; 138(5):1093–100.
20. Lip GYH, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57(2): 173–80.
21. Dans AL, Connolly SJ, Wallentin L, Yang S, Nakamya J, Brueckmann M, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial. Circulation. 2013;127(5):634–40.
Competing interests: Kori Leblanc has participated on advisory boards or received speaker honoraria from Boehringer Ingelheim, Pfizer/BMS, and Bayer, and has participated in research projects with funding from Boehringer Ingelheim and Pfizer/BMS. She is also a member of the Atrial Fibrillation Guidelines Committee of the Canadian Cardiovascular Society. No other competing interests were declared.
Funding: None received.
Canadian Journal of Hospital Pharmacy, VOLUME 68, NUMBER 5, September-October 2015