Mary H H Ensom, BS(Pharm), PharmD, FASHP, FCCP, FCSHP, Clinical Pharmacy Specialist, Diane Décarie, BSc, Research Consultant, Karen Lingertat-Walsh, BScPhm, ACPR, Compounding Pharmacist
To the authors’ knowledge, no data have been published on extemporaneously compounded oral liquid formulations of the nonsteroidal anti-inflammatory drug naproxen.1 This study was undertaken to ascertain the physical and chemical stability of naproxen suspension in dye-free vehicles Oral Mix and Oral Mix SF, after storage at 25°C and 4°C in various types of container for up to 91 days.
Naproxen suspensions (25 mg/mL) were prepared by crushing naproxen 250-mg tablets (Apotex Inc, Weston, Ontario; lot KX4903, expiry June 2016) in 400-mL volumes of each of Oral Mix and Oral Mix SF (Medisca Pharmaceutique Inc, Montréal, Quebec; lot I1114/A, expiry October 2015; lot 57496/D, expiry October 2017, respectively). Each suspension was divided among 6 amber glass bottles (Richards Distribution, Richmond, British Columbia), 6 amber polyethylene terephthalate prescription bottles (Richards Distribution), and twenty-five 3-mL amber oral polypropylene syringes (PreciseDose Dispenser System, Medisca Pharmaceutique Inc; lot 57496/D). For each suspension, 3 glass and 3 plastic bottles were stored at 25°C, and 3 glass and 3 plastic bottles at 4°C. All syringes were stored at 25°C. Glass and plastic bottles were half-filled to allow air space.
All samples were examined for colour, odour, taste, and ease of resuspension on days 0 (baseline), 7, 14, 21, 28, 42, 63, 77, and 91. On each study day, one 3-mL aliquot from each bottle and the contents of 3 syringes from each group were collected to determine pH (pH meter model 800, VWR International, Mississauga, Ontario). Immediately thereafter, a 1.5-mL aliquot from each bottle or syringe was transferred to a threaded, tight-seal cryogenic polypropylene vial (VWR International; lot 1095990) and frozen (−85°C) until analysis by a validated, stability-indicating high-performance liquid chromatography (HPLC) – ultraviolet detection method.2-4
Naproxen stock solutions (25 mg/mL) were prepared from analytical-grade powder (Sigma-Aldrich, Oakville, Ontario; lot SLBH7957V) in HPLC-grade methanol (Fisher Scientific, Whitby, Ontario; lot 148211). The internal standard, ranitidine hydrochloride powder (Sigma-Aldrich; lot MKBQ8277V), was diluted to 1.0 mg/mL in HPLC-grade water (Fisher Scientific; lot 145147). Naproxen standard solutions containing internal standard 0.1 mg/mL were prepared in HPLC-grade methanol to final concentrations of 0.010, 0.016, 0.020, 0.025, 0.028, 0.034, and 0.040 mg/mL, with quality control concentrations of 0.01, 0.018, 0.026, and 0.032 mg/mL. Each sample was passed through a Gelman hydrophilic propylene 13-mm diameter, 0.45-μm microfilter (Acrodisc, Waters Corporation, Mississauga, Ontario; lot 21829235). Standard curves were generated by least-squares regression of the peak area ratio of naproxen to internal standard and the concentration of each naproxen standard.
The HPLC instrumentation (Waters Alliance System model 2690, Waters Corporation) consisted of a delivery pump, automated 200-μL injector, Symmetry Shield RP C18 4.6 × 100 mm column (Waters Corporation; lot 1563433514018), Symmetry Shield RP 3.9 × 20 mm guard column (Waters Corporation; lot 0156342761), and ultraviolet detector (model 2487, Waters Alliance System) set at 232 nm. The mobile-phase gradient consisted of 82% methanol (Fisher Scientific; lot 136336) and 18% 5 mmol/L ammonium formate buffer (Sigma-Aldrich; lot BCBL4456V) at pH 4.0 and room temperature (flow rate 0.8–1.0 mL/min over 4 min).
On the day of analysis, each study sample was thawed and vortex-mixed. A 0.2-mL aliquot was diluted with 1.8 mL of HPLC-grade methanol and centrifuged at 5200 rpm for 5 min. The supernatant (100 μL) was diluted to a final nominal concentration of 0.025 mg/mL in HPLC-grade methanol containing 0.100 mg/mL of internal standard. Each sample (20 μL) was filtered before injection onto the column.
Accelerated degradation of naproxen was achieved as follows. Suspensions of naproxen 5.0 mg/mL in Oral Mix and Oral Mix SF were prepared, mixed (v/v) with 2N hydrochloric acid, 2N sodium hydroxide, 3% hydrogen peroxide, or water, then vortex-mixed and incubated overnight at 60°C. Samples were cooled to room temperature, concentration was adjusted to 0.250 mg/mL in HPLC-grade methanol, and samples were centrifuged, further diluted to 0.025 mg/mL, filtered, and injected onto the column.
Regression analysis demonstrated linearity, with coefficient of determination (r2) of at least 0.996 (n = 4). Intraday and interday coefficients of variation for quality control samples were within acceptable limits (< 10%): 2.64% and 2.63%, respectively, for the 0.010 mg/mL solution; 1.68% and 4.20%, respectively, for the 0.018 mg/mL solution; 1.11% and 1.78%, respectively, for the 0.026 mg/mL solution; and 1.22% and 1.30%, respectively, for the 0.032 mg/mL solution. Mean intraday and interday accuracy values (± standard deviation) were also within acceptable limits (> 90%): 91.63% ± 1.92% and 93.53% ± 2.63%, respectively, for the 0.010 mg/mL solution; 96.39% ± 3.37% and 97.30% ± 3.31%, respectively, for the 0.018 mg/mL solution; 98.34% ± 1.71% and 98.48% ± 1.20%, respectively, for the 0.026 mg/mL solution; and 99.21% ± 0.57% and 99.34% ± 1.38%, respectively, for the 0.032 mg/mL solution.
Retention times were 1.39 min for the internal standard and 2.43 min for naproxen. No interfering peaks were generated by forced degradation. With forced degradation, naproxen peaks decreased by 3.5% to 49.5% in Oral Mix and by 3.7% to 31.1% in Oral Mix SF; minor, non-interfering peaks were observed at 1.44, 1.66, 1.99, 2.25, and 2.75 min. The HPLC method was deemed capable of indicating stability. Time 0 samples stored for 91 days at −85°C showed no degradation products and contained the expected concentration of naproxen (25 mg/mL), which indicated that freezing did not affect stability.
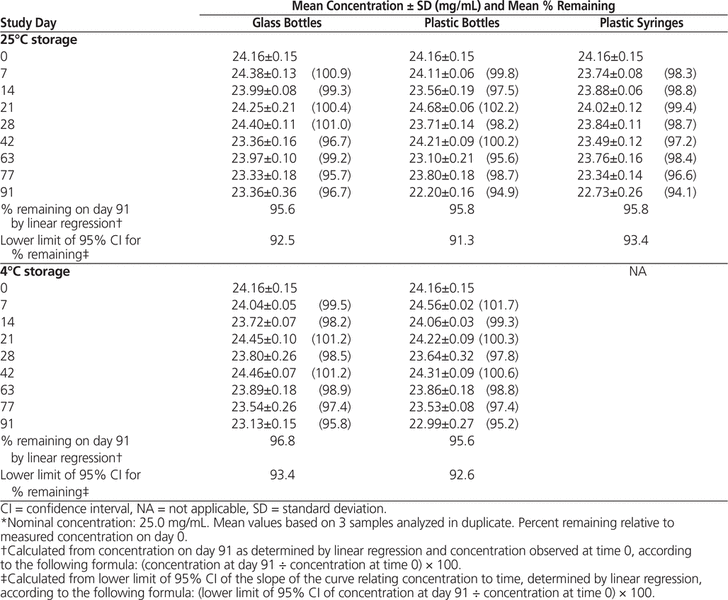
The milky, pale yellow suspensions were easily resuspended throughout the study, with no notable changes in colour, odour, or taste (medium-sweet cherry). The mean pH of suspensions remained stable (range 4.30–4.32 for suspensions in Oral Mix and 4.37–4.39 for suspensions in Oral Mix SF). HPLC analysis showed that all suspensions maintained more than 90% of the original concentration for up to 91 days (Tables 1 and 2).
Table 1. Concentration of Naproxen in Oral Mix Suspension Vehicle During 91 Days of Storage in Glass Bottles, Plastic Bottles, and Plastic Syringes at 25°C and in Glass and Plastic Bottles at 4°C*
Table 2. Concentration of Naproxen in Oral Mix SF Suspension Vehicle During 91 Days of Storage in Glass Bottles, Plastic Bottles, and Plastic Syringes at 25°C and in Glass and Plastic Bottles at 4°C*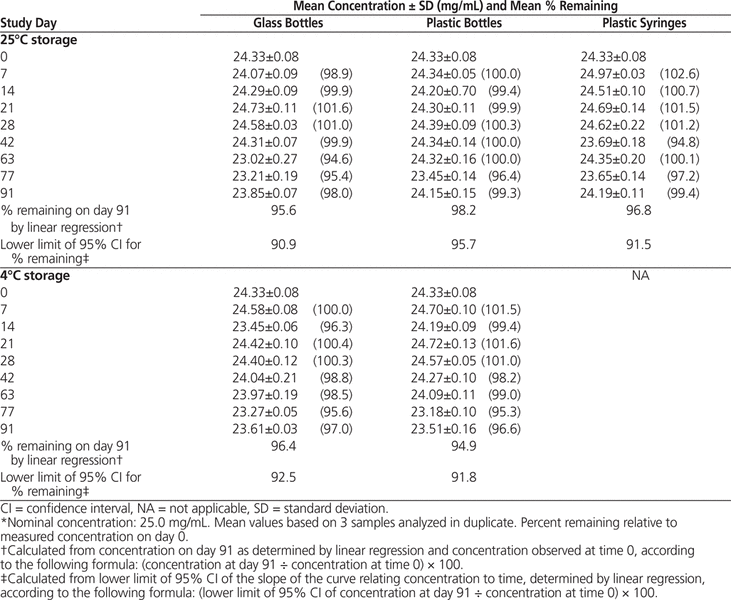
In conclusion, naproxen suspensions (25 mg/mL) in Oral Mix and Oral Mix SF remained stable for up to 91 days when stored in glass and plastic bottles (25°C or 4°C) or syringes (25°C).
1 Trissel L. Trissel’s stability of compounded formulations. 5th ed. Washington (DC): American Pharmacists Association; 2012.
2 Haque T, Talukder MM, Laila S, Fatema K. Development and validation of RP-HPLC method for simultaneous estimation of naproxen and ranitidine hydrochloride. Pak J Pharm Sci. 2010;23(4):379-83.
3 Reddy PS, Saita S, Vasudevmurthya G, Vishwanatha B, Prasada V, Reddy SJ. Stability indicating simultaneous estimation of assay method for naproxen and esomeprazole in pharmaceutical formulation by RP-HPLC. Pharma Chem. 2011;3(6):553-64.
4 Ensom MHH, Decarie D. Stability of thiamine in extemporaneously compounded suspensions. Can J Hosp Pharm. 2005;58(1):26-30.
Mary Ensom is also a Professor in the Faculty of Pharmaceutical Sciences and Distinguished University Scholar, The University of British Columbia, Vancouver, British Columbia. She is the Editor of the CJHP. ( Return to Text )
Funding: This study was funded by an unrestricted educational grant from Medisca Pharmaceutique Inc.
Competing interests: Other than grant support, no competing interests were declared.
Canadian Journal of Hospital Pharmacy, VOLUME 68, NUMBER 6, November-December 2015