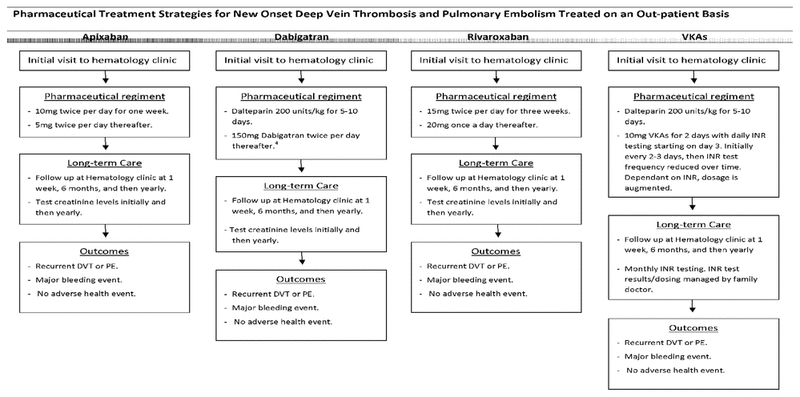
Figure 1 Pharmaceutical-specific treatment strategies. DVT = deep venous thrombosis, INR = international normalized ratio, PE = pulmonary embolism, VKA = vitamin K antagonist.
Abdullah S Al Saleh, Patrick Berrigan, David Anderson, Sudeep ShivakumarABSTRACT
Background
To date, there have been few economic evaluations, from a Canadian perspective, of direct oral anticoagulants (DOACs) for the prevention of recurrent venous thromboembolism (VTE) in patients with acute unprovoked VTE. As a result, there is a lack of consensus about which treatment strategy should be adopted in the clinical setting.
Objectives
To assess the cost-effectiveness of currently approved anti-coagulant options, in terms of cost per quality-adjusted life-year (QALY) gained, for the prevention of recurrent VTE in patients with unprovoked events managed on an outpatient basis.
Methods
Microsoft Excel was used to develop a Markov model. Model parameters were determined using published literature, local hospital data, expert opinion, and chart review. The analysis considered the costs associated with pharmaceuticals, laboratory testing, hematologist fees, and treatment of recurrent VTE and major bleeding events. Effectiveness was measured in terms of QALYs, and incremental cost-effectiveness ratios (ICERs) were calculated.
Results
For treatment lasting 3 months, apixaban represented the most cost-effective DOAC relative to low-molecular-weight heparin (LMWH) + vitamin K antagonist, with an ICER of $7379.66. For 6 months of treatment, apixaban again represented the most cost-effective treatment, with an ICER of $84.08 per QALY gained, and this drug dominated all the other strategies at 12 months. For lifetime treatment, DOACs were unlikely to be cost-effective, given a maximum willingness to pay of $50 000 to $100 000 per QALY. In a probabilistic sensitivity analysis at 6 months, 46.4% of iterations resulted in apixaban having lower costs and better outcomes than LMWH + vitamin K antagonist, and 78.6% of iterations resulted in an ICER below $100 000
Conclusions
The findings of this study suggest that apixaban is likely cost-effective for treatment durations of 3, 6, and 12 months. However, for indefinite treatment, DOACs were unlikely to be cost-effective.
KEYWORDS: economic evaluation, cost-effectiveness, direct oral anticoagulants, venous thromboembolism
RÉSUMÉ
Contexte
À ce jour, on a réalisé peu d’évaluations économiques, d’un point de vue canadien, sur les anticoagulants oraux directs (AOD) utilisés dans la prévention de la thromboembolie veineuse (TEV) récurrente chez les patients atteints de TEV idiopathique aiguë. Pour cette raison, aucun consensus n’a été établi quant à la stratégie thérapeutique à adopter en milieu clinique.
Objectif
Évaluer le rapport coût-efficacité des anticoagulothérapies actuellement approuvées, en ce qui a trait au coût par année de vie pondérée par la qualité (QALY) gagnée, pour la prévention de la TEV récurrente chez les patients ayant subi des événements idiopathiques qui ont été traités en consultation externe.
Méthodes
Le logiciel Excel de Microsoft a servi à créer un modèle de Markov. Les paramètres du modèle ont été établis à l’aide de la littérature, de données de l’hôpital local, d’opinions d’experts et d’une analyse de dossiers médicaux. L’analyse prenait en compte les coûts associés aux médicaments, aux examens de laboratoire, aux honoraires d’hématologues et au traitement de la TEV récurrente et d’hémorragies importantes. L’efficacité était mesurée en nombre de QALY et les rapports coût-efficacité différentiels ont été calculés.
Résultats
Pour un traitement de trois mois, l’apixaban représentait l’AOD offrant le meilleur rapport coût-efficacité comparativement à l’héparine de bas poids moléculaire (HBPM) + un antagoniste de la vitamine K; il présentait un rapport coût-efficacité différentiel de 7379,66 $. Pour un traitement de six mois, l’apixaban représentait à nouveau le traitement le plus efficace par rapport au coût; il présentait un rapport coût-efficacité différentiel de 84,08 $ par QALY gagnée. Ce médicament surclassait toutes les autres stratégies après douze mois de traitement. En ce qui concerne un traitement à vie, les AOD offraient probablement un moins bon rapport coût-efficacité, compte tenu d’une propension à payer maximale se situant entre 50 000 $ et 100 000 $ par QALY. Dans une analyse de sensibilité probabiliste au sixième mois de traitement, 46,4 % des itérations se traduisaient par des coûts moins élevés et de meilleurs résultats pour l’apixaban relativement à l’HBPM + un antagoniste de la vitamine K. De plus, 78,6 % des itérations se traduisaient par un rapport coût-efficacité différentiel de moins de 100 000 $.
Conclusions
Ces résultats laissent croire que l’apixaban présente probablement un rapport coût-efficacité intéressant pour les traitements d’une durée de 3, 6 et 12 mois. Cependant, en ce qui concerne un traitement d’une durée indéterminée, les AOD ne sont sans doute pas avantageux.
MOTS CLÉS: évaluation économique, rapport coût-efficacité, anticoagulants oraux directs, thromboembolie veineuse
Venous thromboembolism (VTE), which comprises deep venous thrombosis (DVT) and pulmonary embolism (PE), is a significant cause of morbidity and mortality worldwide. Standard treatment of VTE, using low-molecular-weight heparin (LMWH) overlapped with vitamin K antagonists (VKAs), is effective but requires frequent laboratory monitoring and has the potential for multiple drug and dietary interactions. In recent years, novel treatment strategies with direct oral anticoagulants (DOACs) have been increasing in popularity and availability. Three DOACs are approved for the treatment of VTE in Canada: dabigatran, rivaroxaban, and apixaban.
This study aimed to assess the cost-effectiveness of currently approved DOACs relative to LMWH + VKA in terms of cost per quality-adjusted life year (QALY) gained when administered to prevent recurrent VTE in patients with unprovoked VTE being managed on an outpatient basis. The analysis took the cost perspective of the health care payer.
Microsoft Excel (Microsoft Corporation, Redmond, Washington) was used to develop a Markov model, a mathematical representation of medical care in which patients transition within a set of health states over simulated time intervals. Using a Markov model allowed the estimation of long-run health outcomes, accounting for patients who transition on and off anticoagulant therapy over time because of VTE recurrence. Determination of the model parameters was based on the published literature, local hospital data, expert opinion, and chart review. The chart review examined the 61 most recent patients who received treatment for acute unprovoked VTE between September 12, 2013, and January 6, 2016, at the Queen Elizabeth II Health Sciences Centre in Halifax, Nova Scotia. Ethics approval was received from the Nova Scotia Health Authority Research Ethics Board, which waived the need for informed consent.
In the study model, a hypothetical cohort of patients received one of the following treatments: apixaban, LMWH in combination with dabigatran (LMWH + dabigatran), rivaroxaban, or LMWH + VKA. Patients in the apixaban group received 10 mg of the drug twice daily for 7 days, followed by 5 mg twice daily thereafter. Patients in the LMWH + dabigatran group received LMWH for 6 days, followed by 150 mg of dabigatran twice daily thereafter. Patients in the rivaroxaban group received 15 mg of the drug twice daily for 21 days, followed by 20 mg daily thereafter. Patients in the LMWH + VKA group received LMWH for 6 days overlapped with VKA. Patients receiving DOACs underwent yearly assessment of renal function by creatinine clearance testing. Patients receiving VKA underwent international normalized ratio (INR) testing to ensure that the drug effect remained within the therapeutic range. INR testing and changes to VKA dosage were initially overseen by a registered nurse specializing in hematology, but once INR remained within the therapeutic range for 2 values, care was transferred to the patient’s family doctor. All patients underwent a complete blood count (CBC) at the start of treatment, and patients taking LMWH underwent a second CBC at 1 week. All patients received consultations with a hematologist initially, then at 1 week and 6 months, and then yearly if they continued anticoagulant therapy (Figure 1). The Markov model assumed 4 health states: recurrent VTE, major bleeding event, death, and no adverse health event (Figure 2).
|
|
||
|
Figure 1 Pharmaceutical-specific treatment strategies. DVT = deep venous thrombosis, INR = international normalized ratio, PE = pulmonary embolism, VKA = vitamin K antagonist. |
||
|
|
||
|
Figure 2 Markov diagram. Recurrent venous thromboembolism (VTE) refers to both deep venous thrombosis (DVT) and pulmonary embolism (PE). LMWH = low-molecular-weight heparin, QALY = quality-adjusted life-years, VKA = vitamin K antagonist. |
||
The model used 6-month Markov cycles over a lifetime follow-up period. Effectiveness was measured with QALY, a metric expressing the extent to which an individual’s health affects length and quality of life.1 The analysis considered costs associated with pharmaceuticals, laboratory testing, hematologist fees, recurrent VTE, and major bleeding events. Incremental cost-effectiveness ratios (ICERs), representing the cost required to gain an additional outcome when comparing 2 interventions, were calculated.2
The reference case used 6 months of anticoagulant therapy, because this represents a frequently used treatment duration in the clinical setting and the most common duration at the study hospital.3 Alternative analyses were also conducted, using 3-month, 12-month, and lifetime treatment durations. These alternative durations were chosen because they are also commonly used to treat patients with unprovoked VTE.3 In addition to the cost-effectiveness analysis, a cost-minimization analysis was conducted, on the assumption that all interventions are equivalent in terms of effectiveness and safety. The Canadian Agency for Drugs and Technologies in Health (CADTH), in its Guidelines for the Economic Evaluation of Health Technologies,4 recommends cost-minimization analysis if the interventions under review are equivalent in terms of effectiveness and safety.
The average age at initial diagnosis was assumed to be 57 years, and the proportion of women was assumed to be 43%. These assumptions were based on patients in studies investigating apixaban, dabigatran, or rivaroxaban referenced by Castellucci and others5 in their systematic review and meta-analysis examining the efficacy and safety of pharmaceuticals used to prevent recurrent VTE.
Costs for apixaban, rivaroxaban, dabigatran, dalteparin (an LMWH), and VKA were taken from the pharmacy at the study hospital and reflect the price paid by patients. The price per day of apixaban was $8.03 for the first 7 days and $4.01 thereafter. The price per day of rivaroxaban was $6.99 for the first 21 days and $3.61 thereafter. The price per day of dabigatran was $4.01. Dosages of LMWH and VKA must be individualized, because a number of factors can influence their therapeutic effect. The chart review showed that patients received on average 18 114.75 units (standard deviation [SD] 8601.84) of dalteparin daily, which corresponds to a cost of $43.10 per day for an average of 5.72 days (SD 1.32 days). To calculate the cost of VKA, care was segmented into 2 periods: the first being the period when dosing is managed by a registered nurse and the second being the period when dosing is managed by the patient’s family doctor. The chart review showed that on average the first period lasted 42.5 days (SD 23.6), with patients receiving an average daily dose of 5.75 mg (SD 2.17 mg), which corresponds to a cost of $0.70 per day. When care was transferred to the family doctor, the patient’s VKA dose for the first period was used to approximate the daily dose of VKA over the second period. The chart review showed that at the time of transfer, patients were receiving on average 5.77 mg (SD 2.76 mg) of VKA daily, which corresponded to a cost of $0.70 per day. A portion of the patients who were receiving LMWH required home care nursing, because they were unable to self-inject. According to previous Canadian economics literature,6,7 a value of 19% was used to estimate the percentage of patients requiring assistance with injections.8 The cost of a home care nursing visit was estimated at $40.00 (Victorian Order of Nurses, personal communication by telephone, December 7, 2015).
The cost of laboratory testing reflects technician wages and the cost of reagents at the study hospital. The costs were $3.85 for each INR test, $2.77 for each CBC, and $1.12 for each creatinine clearance test. The cost of interpreting INR results and adjusting VKA dosage differed depending on the health care provider performing the service. The median compensation to a registered nurse within the hospital was $44.72 per hour, including wages, benefits, and obligatory employer contributions.9 Nurses were interviewed to determine the time required to interpret INR and adjust VKA dosages, and estimated an average of 3 minutes per INR, which corresponds to a cost of $2.24. The Medical Services Insurance (MSI) Physician’s Manual10 lists a general practitioner’s compensation for anticoagulant management as $24.20 per month.
The MSI Physician’s Manual10 lists compensation for an initial consultation with a hematologist as $150.04 and compensation for a follow-up consultation as $66.31.
At the study hospital, most of the patients who experience recurrent VTE receive outpatient care equivalent to care after their initial VTE. However, we estimated that 10% of patients with recurrent VTE require inpatient care. Patients requiring inpatient care receive a daily visit from a hospital-based general practitioner and 8 additional INR tests after discharge, as well as initial and follow-up consultations with a hematologist. According to the Canadian Institute for Health Information,11 the average hospital costs for inpatient DVT and PE care, not including physician fees, are $6730.38 and $7590.18 with average lengths of stay of 6.3 and 5.9 days, respectively. The cost and length of stay for PE represent data for non-DVT embolisms. According to the MSI Physician’s Manual,10 a hospital-based general practitioner receives $31.46 per consultation. Combining the hospital costs of inpatient care, postdischarge INR tests, and general practitioner or hematologist fees results in a total cost of $7192.71 per recurrent DVT and $8039.92 per recurrent PE. For the reference case and the 3- and 12-month scenarios, patients who experienced recurrent VTE after cessation of anticoagulant therapy received 6 months of the same anticoagulant they received after the initial VTE, which would be the minimum duration at the study hospital for a recurrent event, with reassessment at that time for lifetime anticoagulant therapy. Patients receiving lifetime anticoagulant therapy would continue treatment as planned. Costs of major bleeding events were estimated on the basis of McDonald and others,7 who reported costs of $10 904.56 for nonfatal major bleeding events and $40 214.73 for fatal major bleeding events in patients who had undergone hip or knee replacement.12 The cost of fatal recurrent VTE was assumed to be the same as the cost for nonfatal recurrent PE requiring inpatient care. Costs were converted to 2014 Canadian dollars using Statistics Canada’s Consumer Price Index.13
Outcome rates were modelled using 2 time periods, the first being the first Markov cycle after the initial VTE (representing short-run outcome rates) and the second being all successive Markov cycles thereafter (representing long-run outcome rates). If a patient experienced recurrent VTE, the short-run outcome rates were applied to the Markov cycle in which the recurrent VTE occurred, with transition to the long-run outcome rates for Markov cycles thereafter. There was no limit to the number of adverse health events that a patient could experience. Rates of VTE and major bleeding events were determined using the number of events reported in literature. If VTE was not confirmed as the cause of death, the incident was not included in fatality rates for this analysis. Patients who experienced both recurrent DVT and PE were classified as having experienced PE.
To estimate pharmaceutical-specific outcome rates for the first Markov cycle, we pooled the findings of studies presented in Castellucci and others5 investigating apixaban, dabigatran, or rivaroxaban. In the following summary of these studies, the percentage of fatal events is presented in parentheses after rates of recurrent PE and major bleeding events. Agnelli and others14 compared apixaban with LMWH + VKA in a total of 2691 patients, with 0.74% experiencing recurrent DVT, 1.04% (3.57%) experiencing recurrent PE, and 0.56% (6.67%) experiencing a major bleeding event. Schulman and others15,16 compared LMWH + dabigatran with LMWH + VKA in 1274 and 1279 patients, respectively. Combining the findings of these 2 studies yielded a recurrent DVT rate of 1.61%, a recurrent PE rate of 0.94% (16.67%), and a major bleeding event rate of 1.37% (2.86%). Bauersachs and others17 and Büller and others18 compared rivaroxaban with LMWH + VKA in 2419 and 1731 patients, respectively. Combining the findings of these studies yielded a recurrent DVT rate of 0.77%, a recurrent PE rate of 1.11% (6.52%), and a major bleeding event rate of 0.96% (7.50%). To estimate outcome rates for LMWH + VKA, we pooled the findings of studies presented in Castellucci and others5 investigating apixaban, dabigatran, or rivaroxaban and using LMWH + VKA as the control intervention,14–18 which resulted in a recurrent DVT rate of 1.20%, a recurrent PE rate of 0.94% (6.82%), and a major bleeding event rate of 1.78% (7.19%) in a total of 9389 patients.
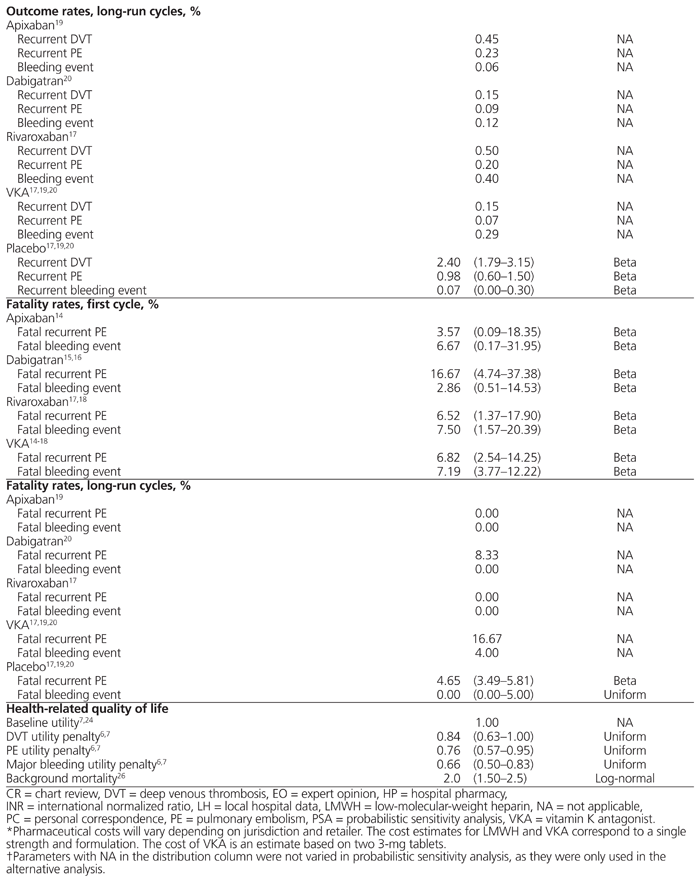
To estimate pharmaceutical-specific outcome rates for long-run Markov cycles, we pooled the findings of studies investigating extended-use DOACs to prevent recurrent VTE identified in a literature review.17,19,20 Long-run outcome rates were converted to reflect 6-month rates. Agnelli and others19 compared apixaban with placebo in a total of 813 patients, with 0.45% experiencing recurrent DVT, 0.23% (0%) experiencing recurrent PE, and 0.06% (0%) experiencing a major bleeding event. Schulman and others20 compared dabigatran with VKA in 1430 patients and compared dabigatran with placebo in 681 patients. Combining the findings of these 2 studies yielded a recurrent DVT rate of 0.15%, a recurrent PE rate of 0.09% (8.33%), and a major bleeding event rate of 0.12% (0%). Bauersachs and others17 compared rivaroxaban with placebo in a total of 602 patients, with 0.50% experiencing recurrent DVT, 0.20% (0%) experiencing recurrent PE, and 0.40% (0%) experiencing a major bleeding event. To estimate long-run outcome rates in patients receiving VKA, we used the findings of Schulman and others,20 who used VKA in 1426 patients in the control arm of their study. Schulman and others20 reported 0.15% experiencing recurrent DVT, 0.07% (16.67%) experiencing recurrent PE, and 0.29% (4.00%) experiencing a major bleeding event.
To approximate long-run outcome rates in patients who discontinued anticoagulant therapy in the reference case and the 3-month and 12-month scenarios, we used the outcome rates for patients receiving placebo in the extended trials.17,19,20 Combining the results of these studies yielded a recurrent DVT rate of 2.40%, recurrent PE rate of 0.98% (4.65%), and major bleeding event rate of 0.07% (0%) in a total of 2085 patients.
On the basis of previous Canadian economic literature,6,7 we applied utility scores of 0.84 for recurrent DVT, 0.76 for recurrent PE, and 0.66 for major bleeding events.21–23 Disutility associated with adverse health events was assumed to last for 3 months,6,7 and a healthy patient was assumed to have a utility score of 1.6,7,24
Background mortality was estimated by applying a standardized mortality ratio of 2.0 to the expected mortality in the general Canadian population.25 The standardized mortality ratio of 2.0 was chosen based on the findings of a cohort study investigating long-run mortality in VTE patients.26
Cost and QALY were discounted at a rate of 5%, and sensitivity analyses were conducted with rates of 0% and 3%. In the reference case and alternative analyses, costs and QALY were aggregated for 25 years, representing a lifetime follow-up period.25
One-way sensitivity analysis was conducted using the ICER associated with the upper and lower bounds of the probabilistic sensitivity analysis (PSA) intervals for key parameters (Table 1). Each DOAC was compared with LMWH + VKA in the reference case, and the results are presented in a tornado plot.
Table 1 Parameter Estimates, PSA Intervals, and Distributions*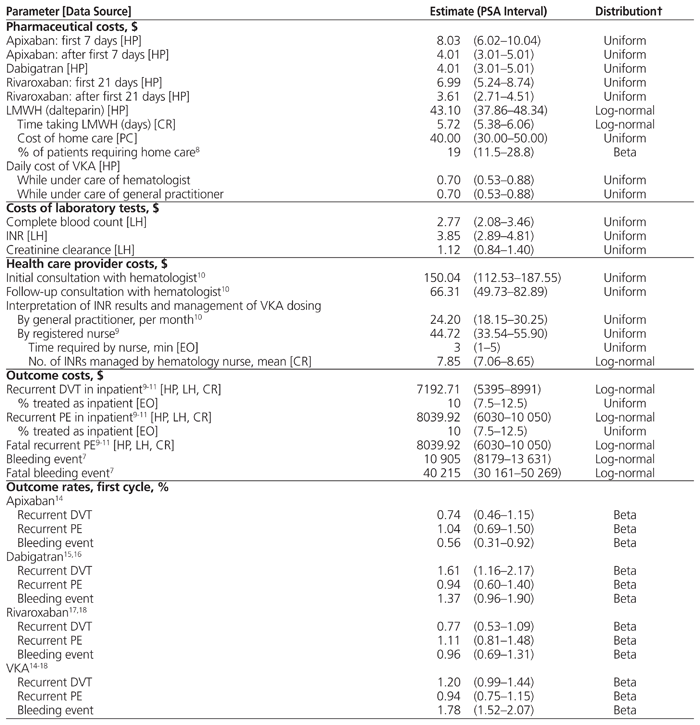
PSA was conducted by simultaneously varying all parameter estimates in the reference case within relevant intervals, subject to statistical distributions. Once each parameter value was varied, the subsequent ICER was recorded. This process was repeated 10 000 times. Parameter intervals and distributions used in creating the model are presented in Table 1.
PSA intervals for the percentage of patients requiring home care and outcome rates represented 95% confidence intervals taken from the literature or calculated using the method of Clopper and Pearson.27 The intervals related to pharmaceutical costs (with the exception of LMWH), laboratory testing, health care provider reimbursements, health outcome costs, standardized mortality ratio, and health-related quality of life represent ±25% of the parameter value estimate. PSA intervals for LMWH and the average number of INR tests managed by a registered nurse represent 95% confidence intervals determined from the chart review.
To assess internal validity, the literature-derived parameter estimates used to inform the model for key outcomes were compared with the model’s predicted values for the corresponding outcomes. To assess external validity, values observed in the literature for key outcomes were compared with those predicted by the model.28–30
In terms of internal validation, model predictions were similar to the literature-derived parameter estimates (Table 2A). In terms of external validation, the model predicted similar mortality rates to those observed in previous literature28,30 but higher rates of recurrent VTE28 (Table 2B).
Table 2A Internal Validation of Model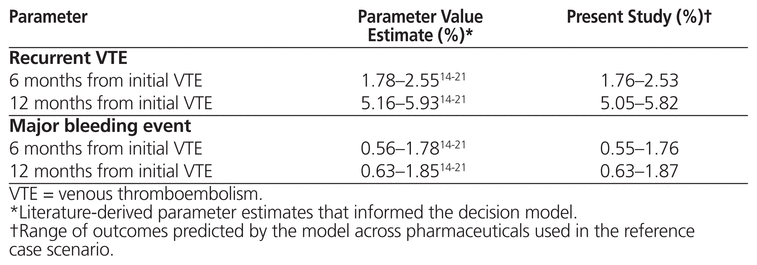
Table 2B External Validation of Model (Probability of Occurrence)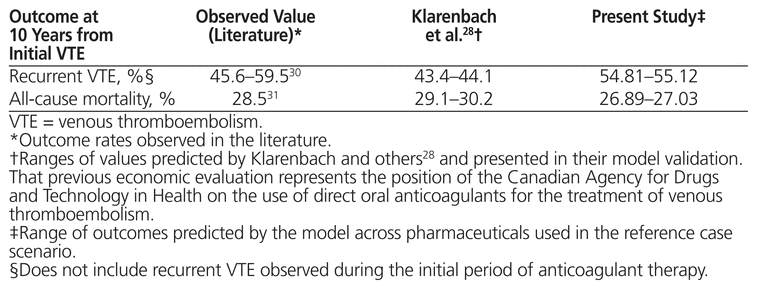
In the reference case, apixaban dominated the other DOACs (i.e., had superior costs and outcomes) and had an ICER of $84.08 relative to LMWH + VKA (Table 3). A discount rate of 0% resulted in apixaban dominating all other strategies, and a discount rate of 3% resulted in apixaban dominating the other DOACs and having an ICER of $36.79 relative to LMWH + VKA (Table 3).
Table 3 Cost-Effectiveness Results for Reference Case and Alternative Models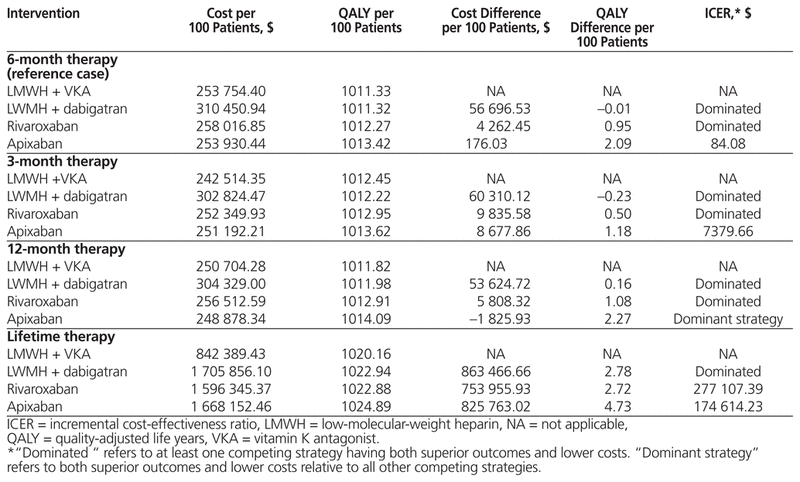
Alternative analyses were conducted using 3-month, 12-month, and lifetime durations of anticoagulant therapy. For 3-month duration, apixaban dominated the other DOACs and had an ICER of $7379.66 relative to LMWH + VKA. For 12-month duration, apixaban dominated all other strategies. For lifetime duration, apixaban had the lowest ICER of any DOAC, at $174 614.23 (Table 3).
This cost-minimization analysis found that LMWH + VKA was the least costly strategy when considering non–outcome-related costs for treatment duration lasting 6 months or longer. For 3-month treatment durations, DOACs not requiring LMWH were the least costly.
One-way sensitivity analysis suggested that short-run fatality rates and pharmaceutical costs were the largest drivers of uncertainty in the current analysis (Figure 3).
|
|
||
|
Figure 3 Tornado plot of modelling results. This plot depicts the range of values of incremental cost-effectiveness ratio that result from varying a single model input over a given interval. It can be used to infer which model inputs have the greatest influence on cost-effectiveness. HRQoL = health-related quality of life, LMWH = low-molecular-weight heparin, MBE = major bleeding event, VKA = vitamin K antagonist, VTE = venous thromboembolism. |
||
Using the 10 000 PSA iterations, a cost-effectiveness acceptability curve was constructed (Figure 4). This type of curve uses PSA iterations to provide decision-makers with a degree of certainty regarding an intervention’s cost-effectiveness relative to another intervention. The curve depicts the percentage of PSA iterations that fall below a given willingness-to-pay for the outcome of interest. Figure 5 shows the cost-effectiveness plane, plotting the change in QALY on the horizontal axis versus the change in cost on the vertical axis for each of the 10 000 iterations. Decision-makers can use the cost-effectiveness plane to gain additional insight into an intervention’s cost-effectiveness, by examining the quadrants into which the bulk of PSA iterations fall. The upper quadrants reflect higher costs, and quadrants on the right represent superior outcomes associated with a new intervention (in Figure 5, apixaban is the new intervention). The cost-effectiveness acceptability curve compared apixaban with LMWH + VKA in the reference case: 46.4% of iterations resulted in apixaban dominating LMWH + VKA; 78.6% of iterations resulted in ICER below $100 000; 97.1% of iterations resulted in apixaban generating more QALY; and 47.2% of PSA iterations resulted in apixaban being cost-saving.
|
|
||
|
Figure 4 Cost-effectiveness acceptability curve for apixaban versus low-molecular-weight heparin overlapped with vitamin K antagonist. QALY = quality-adjusted life-years. |
||
|
|
||
|
Figure 5 Cost-effectiveness plane for apixaban versus low-molecular-weight heparin overlapped with vitamin K antagonist. The plane represents cost-effectiveness per 100 patients. QALY = quality-adjusted life-years. |
||
Few Canadian economic evaluations have compared currently approved DOACs with LMWH + VKA for the treatment of unprovoked VTE. Klarenbach and others28 compared DOACs with LMWH + VKA and reported that the ICERs associated with DOACs were too high to be considered attractive. Quon and others31 compared LMWH + dabigatran, rivaroxaban, and LMWH + VKA with apixaban for extended treatment in preventing recurrent VTE and found that apixaban was the dominant strategy for 3- and 6-month durations of anticoagulant therapy and was a cost-effective alternative to LMWH + VKA for 18-month and lifetime durations of anticoagulant therapy.
Klarenbach and others28 presented CADTH’s position on the cost-effectiveness of DOACs for the treatment of VTE. Because CADTH is Canada’s national authority on the economic evaluation of health technologies, it is important to discuss how differences in model assumptions between the current study and that of Klarenbach and others28 may have led to differing results. From a cost perspective, the most important difference between the current study and the existing literature was the application of a pharmaceutical cost of $0.70 per day for VKA, rather than the $0.11 used in previous studies.28,31 With respect to effectiveness, the current study found that DOACs (most notably apixaban) were more effective at generating QALY relative to LMWH + VKA than was the case in the previous literature.28,31 This difference probably stems from the assumption in the current study that adverse health events affect patients’ utility scores to a greater extent than was assumed in the previous literature. The net effect of these discrepancies is that the current study found apixaban to be more cost-effective than was reported by Klarenbach and others.28
In terms of internal validation, the model predictions in the current study were similar to the literature-derived parameter estimates. In terms of external validation, the model predicted a wider range of recurrent VTE than Klarenbach and others.28 Although we acknowledge this discrepancy, our values fell within the 95% confidence intervals reported in a previous study for the population with unprovoked VTE population.29 Given that the population with unprovoked VTE was the primary focus of the current study, we concluded that the model was reasonable in its predictions.
One-way sensitivity analysis suggested that short-run fatality rates were the largest drivers of uncertainty in the current analysis. We attributed this uncertainty to small sample sizes, relative to fatality rates, in the previous literature,14–20 which resulted in large PSA intervals for these parameters. Additionally, one-way sensitivity analysis suggested that cost-effectiveness results were sensitive to changes in pharmaceutical costs. Subsequently, modest changes in drug prices could yield significantly different cost-effectiveness results. Decision-makers should consider relative price differences between pharmaceuticals, if applying these results to their own jurisdictions.
This analysis had several limitations. The length of follow-up and the ratio of patients with unprovoked VTE to those with provoked VTE were not uniform across the studies used to determine outcome rates.14–20 We suspect that the net effect of heterogeneity in the literature informing the current study resulted in understatement of the cost-effectiveness of DOACs. The costs of major bleeding events and recurrent VTE were derived from studies in which patients were using VKAs, and there is uncertainty about the cost of such events for patients using DOACs. Currently, there is inadequate sample size in the existing literature to establish superiority, in terms of efficacy or safety, among various anticoagulant therapy strategies. Future economic analyses would benefit from additional safety and efficacy research. Outcome rates were converted to reflect treatment durations of 6 months, which required assumptions about how outcome events reported in literature would be distributed over the study’s follow-up period. This study did not incorporate indirect costs such as missed time at work. We speculate that DOACs would reduce such indirect costs, because they are associated with less patient monitoring than LMWH + VKA.
These findings suggest that apixaban is likely cost-effective for treatment durations of 3, 6, and 12 months. However, for lifetime treatment duration, DOACs are unlikely to be cost-effective, because ICERs were significantly above a standard maximum willingness-to-pay per QALY of $50 000 to $100 000.32 Given that the use of DOACs for treatment of VTE is increasing, these findings may help to shape future reimbursement policies.
1 Weinstein MC, Torrance G, McGuire A. QALYs: the basics. Value Health. 2009;12 Suppl 1:S5–9.
2 Detsky AS, Naglie IG. A clinician’s guide to cost-effectiveness analysis. Ann Intern Med. 1990;113(2):147–54.
3 Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl): e419S–e496S.

4 Guidelines for the economic evaluation of health technologies: Canada. 3rd ed. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2006.
5 Castellucci LA, Cameron C, Le Gal G, Rodger MA, Coyle D, Wells PS, et al. Clinical and safety outcomes associated with treatment of acute venous thromboembolism: a systematic review and meta-analysis. JAMA. 2014;312(11):1122–35.
6 Diamantopoulos A, Lees M, Wells PS, Forster F, Ananthapavan J, McDonald H. Cost-effectiveness of rivaroxaban versus enoxaparin for the prevention of postsurgical venous thromboembolism in Canada. Thromb Haemostasis. 2010;104(4):760–70.
7 McDonald H, Diamantopoulos A, Wells P, Lees M, Folkerts K, Forster F, Ananthapavan J. Cost-effectiveness of rivaroxaban in the prevention of venous thromboembolism: a Canadian analysis using the Ontario Ministry of Health perspective. J Med Econ. 2012;15(5):817–28.
8 Harrison L, McGinnis J, Crowther M, Ginsberg J, Hirsh J. Assessment of outpatient treatment of deep-vein thrombosis with low-molecular-weight heparin. Arch Intern Med. 1998;158(18):2001–3.
9 Collective agreement between the Capital District Health Authority and the Nova Scotia Government and General Employees Union: Healthcare Bargaining Unit. Halifax (NS): Nova Scotia Health Authority; 2014 [cited 2015 Aug 6]. Available from: www.nshealth.ca/sites/nshealth.ca/files/central_zone_collective_agreements_health_care_collective_agreement_2011-2014.pdf
10 Physician’s manual. Halifax (NS): Nova Scotia Medical Services Insurance; 2014 [cited 2015 Dec 4]. Available from: www.medavie.bluecross.ca/static/MSI/PhysicianManual.pdf
11 Patient cost estimator. Ottawa (ON): Canadian Institute for Health Information; [cited 2015 Dec 24]. Available from: https://www.cihi.ca/en/spending-and-health-workforce/spending/patient-cost-estimator
12 Ontario case costing initiative (OCCI). Acute inpatient databases for 2005/06 and 2006/07. Toronto (ON): Ministry of Health and Long-Term Care; 2007.
13 Table 326-0021: Consumer price index. In: CANSIM [database]. Ottawa (ON): Statistics Canada.; [cited 2015 Mar 10]. Available from: www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/econ46a-eng.htm
14 Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al.; AMPLIFY Investigators. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808.
15 Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thrombo-embolism. N Engl J Med. 2009;361(24):2342–52.
16 Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–72.
17 EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–510.
18 EINSTEIN-PE Investigators; Büller HR, Prins MH, Lensing AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–97.
19 Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al.; AMPLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699–708.
20 Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al.; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709–18.
21 Haentjens P, De Groote K, Annemans L. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. A cost-utility analysis. Arch Orthop Traum Surg. 2004;124(8):507–17.
22 Gould MK, Dembitzer AD, Sanders GD, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis: a cost-effectiveness analysis. Ann Intern Med. 1999;130(10):789–99.
23 Lenert LA, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep vein thrombosis. J Am Med Inform Assoc. 1997;4(1):49–56.

24 Mahmoudi M, Sobieraj DM. The cost-effectiveness of oral direct factor Xa inhibitors compared with low-molecular-weight heparin for the prevention of venous thromboembolism prophylaxis in total hip or knee replacement surgery. Pharmacotherapy. 2013;33(12):1333–40.
25 Life tables, Canada, provinces and territories: 2010 to 2012 [database online]. Ottawa (ON): Statistics Canada; 2016 [cited 2017 Jan 12]. Available from: www.statcan.gc.ca/pub/84-537-x/84-537-x2016006-eng.htm
26 Flinterman LE, van Hylckama Vlieg A, Cannegieter SC, Rosendaal FR. Long-term survival in a large cohort of patients with venous thrombosis: incidence and predictors. PLoS Med. 2012;9(1):e1001155.

27 Clopper C J, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–13.
28 Klarenbach S, Lee K, Boucher M, So H, Manns B, Tonelli M. Direct oral anticoagulants for the treatment of venous thromboembolic events: economic evaluation. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016.
29 Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92(2):199–205.
30 Schulman S, Lindmarker P, Holmström M, Lärfars G, Carlsson A, Nicol P, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost. 2006;4(4):734–42.
31 Quon P, Le HH, Raymond V, Mtibaa M, Moshyk A. Clinical and economic benefits of extended treatment with apixaban for the treatment and prevention of recurrent venous thromboembolism in Canada. J Med Econ. 2016;19(6):557–67.
32 Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19(4):422–37.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 70, NUMBER 3, May-June 2017