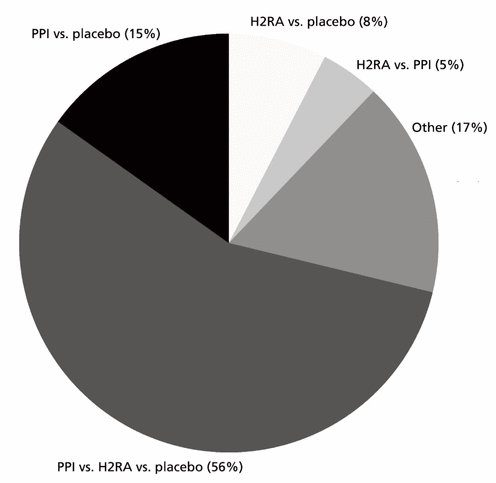
Figure 1 Interventions to be tested in a future randomized controlled trial of stress ulcer prophylaxis, as reported by survey respondents (n = 66).
Mark Duffett, Karen Choong, Jennifer Foster, Elaine Gilfoyle, Jacques Lacroix, Deborah J CookABSTRACT
Background
Stress ulcer prophylaxis is commonly used in pediatric critical care, to prevent upper gastrointestinal bleeding. The most frequently used agents are histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs). The risk–benefit ratio for stress ulcer prophylaxis is uncertain, because data from randomized clinical trials (RCTs) on the effectiveness and harms of prophylaxis in children are limited.
Objective
To describe the views of Canadian pediatric intensivists about a future RCT of stress ulcer prophylaxis.
Methods
We conducted an online survey of Canadian pediatric critical care physicians. We e-mailed information about the study and a link to a 10-item survey to 111 potential respondents, with 2 reminders for nonrespondents. We assessed the relationship between respondents’ characteristics and their views about the need for and potential participation in a trial using logistic regression and assessed regional differences using the χ2 test.
Results
The 68 physicians who replied (61% of potential respondents) had a median of 12 (interquartile range 5–20) years of experience. Forty-four (65%) of the respondents stated that a large, rigorous RCT of stress ulcer prophylaxis in children is needed, and 94% (62 of 66) indicated that it should include a placebo group. The 3 most common designs suggested were a 3-arm trial comparing PPI, H2RA, and placebo (56% [37 of 66 respondents to this question]) and 2-arm trials comparing PPI with placebo (15% [n = 10]) and H2RA with placebo (8% [n = 5]). The 5 patient groups that respondents most commonly stated should be excluded (because they should not receive placebo) were children receiving acid suppression at home (66% [42 of 64 respondents to this question]) or corticosteroids (59% [n = 38]), those with severe coagulopathy or receiving extracorporeal membrane oxygenation (both 36% [n = 23]), and those with burns (31% [n = 20]). Most respondents indicated a willingness to participate in an RCT (64% [42 of 66 respondents to this question]), whereas some (29% [n = 19]) indicated that participation would depend on trial design or funding; only 8% (n = 5) were disinclined to participate.
Conclusions
There is considerable interest in a placebo-controlled RCT of stress ulcer prophylaxis among pediatric critical care physicians in Canada, but consensus on key elements of the trial design is needed.
KEYWORDS: pediatric critical care, survey, stress ulcer prophylaxis, randomized controlled trial
RÉSUMÉ
Contexte
La prophylaxie de l’ulcère de stress est fréquemment employée en soins intensifs pédiatriques afin de prévenir les saignements du tractus gastro-intestinal supérieur. Les agents les plus souvent employés sont les antagonistes des récepteurs H2 de l’histamine (anti-H2) et les inhibiteurs de la pompe à protons (IPP). Le rapport bénéfice-risque pour la prophylaxie de l’ulcère de stress est incertain, car les données provenant d’essais cliniques à répartition aléatoire (ECRA) sur l’efficacité et les dangers de la prophylaxie chez l’enfant sont peu nombreuses.
Objectif
Décrire les points de vue des pédiatres intensivistes canadiens à propos d’un futur ECRA sur la prophylaxie de l’ulcère de stress.
Méthodes
Nous avons réalisé un sondage en ligne auprès de médecins canadiens en soins intensifs pédiatriques. Nous avons envoyé par courriel de l’information sur l’étude, dont un lien menant au sondage en dix points, à 111 répondants potentiels et deux rappels à ceux n’ayant pas répondu d’emblée. Nous avons évalué, au moyen d’un modèle de régression logistique, les points de vue des répondants à propos de la nécessité d’un essai et de leur participation potentielle à celui-ci en fonction de leurs caractéristiques individuelles et, à l’aide d’un test de χ2, les différences régionales.
Résultats
Les 68 médecins (61 % des répondants potentiels) qui ont répondu avaient une médiane de 12 (écart interquartile de 5 à 20) années d’expérience. Quarante-quatre (65 %) des répondants indiquaient qu’il serait nécessaire de procéder à un important et rigoureux ECRA sur la prophylaxie de l’ulcère de stress chez l’enfant et 94 % (62 des 66 répondants) indiquaient que l’étude devrait comprendre un groupe placebo. Les trois plans expérimentaux les plus souvent suggérés étaient : un essai à trois groupes comparant les IPP, les anti-H2 et le placebo (56 % [37 des 66 répondants à cette question]), un essai à deux groupes comparant les IPP au placebo (15 % [n = 10]) et un essai à deux groupes comparant les anti-H2 au placebo (8 % [n = 5]). Les cinq groupes de patients que les répondants indiquaient le plus souvent comme ceux devant être exclus (parce qu’ils ne devraient pas recevoir de placebo) étaient : les enfants recevant un traitement de suppression de la sécrétion d’acide à la maison (66% [42 des 64 répondants à cette question]), ceux traités à l’aide de corticoïdes (59 % [n = 38]), ceux atteints d’une coagulopathie grave ou ceux traités par oxygénation extracorporelle sur oxygénateur à membrane (36 % chacun [n = 23]) et ceux souffrant de brûlures (31% [n = 20]). La plupart des répondants ont indiqué être prêts à participer à un ECRA (64% [42 des 66 répondants à cette question]), alors que certains (29 % [n = 19]) ont indiqué que leur participation dépendrait du plan de l’étude ou de son financement; seulement 8% (n = 5) n’étaient pas enclins à participer.
Conclusions
On constate parmi les médecins canadiens en soins intensifs pédiatriques un intérêt marqué pour la tenue d’un ECRA comparatif contre placebo sur la prophylaxie de l’ulcère de stress, mais il est d’abord nécessaire d’obtenir un consensus sur les éléments clés du plan expérimental.
KEYWORDS: soins intensifs pédiatriques, sondage, prophylaxie de l’ulcère de stress, essai clinique à répartition aléatoire
Despite very weak evidence to support the efficacy of stress ulcer prophylaxis, most critically ill children receive medications to reduce gastric acid secretion. In a US study of 42 hospitals, 60% of children admitted to pediatric intensive care units (PICUs) received acid suppression, most commonly with proton pump inhibitors (PPIs) or histamine-2 receptor antagonists (H2RAs).1 In a prospective observational study of 398 children from 5 PICUs in Brazil, 78% received prophylaxis.2
There is currently insufficient evidence to assess the benefits of routine prophylaxis. The 3 published trials that have reported macroscopic or important bleeding (340 children in total)3–5 found no difference between children receiving prophylaxis and those receiving no prophylaxis (relative risk 0.71, 95% confidence interval [CI] 0.42–1.19, p = 0.19).6 The confidence interval around this summary estimate is wide and the strength of inference is low, because there were only 21 bleeding events. Furthermore, the use of prophylaxis carries certain risks. For example, acid suppression in critically ill children is independently associated with ventilator-associated pneumonia (odds ratio [OR] 2.0, 95% CI 1.2–3.6, p = 0.01),7 and accumulating data in other populations confirm an increased risk of infections with PPI use.8 There is clearly uncertainty about this issue, and a large, multicentre randomized clinical trial (RCT) is the best way to resolve this.
Such an RCT would be challenging to complete. Only 320 RCTs in pediatric critical care have been published. They have typically been small (median sample size 50 children, with 78% of the trials involving a single centre), and 30% were stopped early (86% of these because of futility or poor recruitment).9 More specifically, recruitment for an RCT of stress ulcer prophylaxis would be challenging. The feasibility of a future trial will depend on the willingness of centres to participate, as well as individual physician equipoise and willingness to adhere to the study protocol. The RCT must also be designed so that its results will be clinically relevant: the population, intervention, and outcomes should otherwise reflect current practice. Careful planning to ensure that a trial is feasible and produces clinically relevant results is the scientifically and ethically responsible approach.
The objective of this survey was to describe the views of Canadian pediatric intensivists on a future trial of stress ulcer prophylaxis in critically ill children.
The domains of interest for this survey were the demographic characteristics of the respondents; their views on the need for, and optimal design of, an RCT of stress ulcer prophylaxis in children; and their interest in participating in an RCT. For this survey, we adapted a questionnaire from a similar survey in adult critical care.10 The brief questionnaire (Appendix 1, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/122/showToc) included a mixture of closed- and open-ended questions. Five pediatric critical care clinicians and researchers who were not involved as investigators reviewed the pediatric survey instrument for clarity and relevance, and the instrument was revised on the basis of their assessments. The items for the original adult survey were generated using literature review, e-mail and telephone correspondence among the investigators, and consensus discussion. The survey was pilot tested by 5 intensive care physicians, and its clinical sensibility was evaluated by 8 clinicians with expertise in survey methods and research methods in critical care.
Our population of interest was Canadian pediatric critical care attending physicians. Trainees were not eligible to participate. We contacted a representative from each of the 16 centres in Canada with a PICU to confirm the names and e-mail addresses of the physicians at the site, which allowed updating of an e-mail list used in a previous national pediatric survey.11
We sent a personalized e-mail message, with a link to the electronic questionnaire, to each of the 111 potential respondents in February 2016 with 2 reminders to nonrespondents at approximately 2-week intervals. We did not provide an incentive for participation. This survey was approved by the Hamilton Integrated Research Board; we deemed completion of the questionnaire to indicate respondents’ consent to participate.
For description of the survey respondents, we reported continuous data as medians (with interquartile range [IQR]) and binary data as counts (with percentages). For all analyses, we used the actual number of respondents as the denominator. To summarize the answers to open-ended questions, we grouped similar responses into themes. To assess regional differences, we grouped respondents into 3 regions (Ontario with the Atlantic provinces, Quebec, and Western Canada), and used the χ2 test to compare the proportion of respondents in each region reporting that a trial was needed and that they would be interested in participating in a trial. Finally, we used logistic regression to assess the independent relation between respondent characteristics (years of critical care experience and region) and responses (reporting that a trial was needed and interest in participating in a trial), with results reported as the odds ratio (OR), its associated 95% confidence interval (CI), and p value. We used R software, version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria) to conduct the analyses, with α = 0.05 as the criterion for statistical significance.
Sixty-eight physicians (61% of the 111 potential respondents) participated in the survey (23 from Ontario and Atlantic Canada, 19 from Quebec, and 26 from Western Canada) with at least 1 respondent from each of the 16 PICUs in Canada. Participants’ median duration of experience in pediatric critical care was 12 years (IQR 5–20 years).
Forty-four (65%) of the respondents stated that a large, rigorous randomized trial of stress ulcer prophylaxis in critically ill children is needed. The most frequent reasons given by the 36 respondents who provided a specific rationale for the need for a future trial were inadequacy of current evidence (75% [n = 27]), wide variation in practice (22% [n = 8]), frequency of use of stress ulcer prophylaxis (22% [n = 8]), and existence of equipoise (14% [n = 5]). The most frequent reasons given by the 24 respondents (35% of all respondents) who stated that a trial is not needed were the low risk of bleeding (54% [n = 13]), difficulty or cost of conducting such a trial (29% [n = 7]), low priority of the trial question (17% [n = 4]), infrequency of use of stress ulcer prophylaxis (17% [n = 4]), clinicians’ lack of equipoise (17% [n = 4]), and low cost of the intervention (8% [n = 2]). There was no difference in the perceived need for a trial among regions: for Ontario and Atlantic, 61% (n = 14); for Quebec, 74% (n = 14); and for Western Canada, 62% (n = 16) (p = 0.63). Logistic regression showed that neither years of experience (OR 0.7, 95% CI 0.3–1.3, p = 0.20) nor region (for Quebec, OR 2.0, 95% CI 0.5–8.8, p = 0.33; for Western Canada, OR 0.9, 95% CI 0.3–3.1, p = 0.92 [relative to Ontario and Atlantic Canada]) was independently associated with perceived need for a trial of stress ulcer prophylaxis in critically ill children.
The preferred intervention and comparators for a future trial are presented in Figure 1. Sixty-two of 66 respondents (94%) indicated that a future trial should include a placebo group, and 55 of 66 (83%) preferred some permutation of PPI, H2RA, and placebo. Figures 2 and 3 show perceived indications for stress ulcer prophylaxis and perceived contraindications to receiving placebo, respectively. The most frequently selected indication for starting stress ulcer prophylaxis was membership in a specific subpopulation (Figure 2), with the 5 most frequently named subpopulations being patients receiving corticosteroids (41% [n = 26 of 64 respondents to this question]); patients with traumatic brain injury, extracorporeal membrane oxygenation, or burns (17% [n = 11] each); and patients with coagulopathy (14% [n = 9]).
|
|
||
|
Figure 1 Interventions to be tested in a future randomized controlled trial of stress ulcer prophylaxis, as reported by survey respondents (n = 66). |
||
|
|
||
|
Figure 2 Patients who should typically receive stress ulcer prophylaxis, as reported by survey respondents (n = 64). Some respondents chose more than 1 option. “Non-invasive” = receiving non-invasive ventilation, NPO = nothing by mouth, “2 days” = 2 days or more. |
||
|
|
||
|
Figure 3 Patients who should not receive placebo, and thus should be excluded from future randomized, placebo-controlled trials of stress ulcer prophylaxis, as reported by survey respondents (n = 68). Some respondents chose more than 1 option. H2RA = histamine-2 receptor antagonist, NPO = nothing by mouth, PPI = proton pump inhibitor. |
||
Reasons for stopping stress ulcer prophylaxis, as identified by survey respondents, are presented in Figure 4. Here, enteral feeding was important: the 2 most commonly selected reasons reported for stopping stress ulcer prophylaxis were receiving any feeds or receiving full feeds.
|
|
||
|
Figure 4 Triggers to stop stress ulcer prophylaxis (assuming the patient has no signs of bleeding), as reported by survey respondents (n = 65). Some respondents chose more than 1 option. PICU = pediatric intensive care unit. |
||
Most respondents (64% [42 of 66 respondents to this question]) reported that they would be interested in participating in such a trial. Only 8% (n = 5) were not interested. The remaining 29% (n = 19) reported that their interest in participating would depend on other factors, most frequently trial design (23% [n = 15]). Willingness to participate did not differ significantly by region (Ontario and Atlantic, 52% [n = 12]; Quebec, 79% [n = 15]; Western Canada, 58% [n = 15]; p = 0.18). Logistic regression showed that neither years of experience (OR 0.9, 95% CI 0.9–1.0, p = 0.09) nor region (for Quebec, OR 4.4, 95% CI 1.1–22.1, p = 0.05; for Western Canada, OR 1.2, 95% CI 0.4–3.9, p = 0.76 [relative to Ontario and Atlantic Canada]) was independently associated with interest in participating in a future RCT.
In this self-administered survey of Canadian pediatric critical care physicians, most respondents (65%) stated that a large rigorous RCT of stress ulcer prophylaxis in children is needed, and 64% reported willingness to enroll patients in such an RCT. Regarding the design, 94% stated that the trial should include a placebo group, and the design most commonly suggested (by 56%) was a 3-arm trial comparing PPI, H2RA, and placebo. No clear majority opinion emerged on trial inclusion and exclusion criteria or the timing of drug cessation.
A recent survey of adult intensivist members of the Canadian Critical Care Trials Group found broad support for an RCT of stress ulcer prophylaxis.10 When asked specifically about their attitudes toward a future trial comparing PPI with placebo, 85% endorsed the need for such a trial — higher than the 65% in this survey. Members of a clinical trials group may be more favourably disposed toward randomized trials in general, but there are also important differences between adults and children in the published evidence and in the risks of bleeding, ventilator-associated pneumonia, and Clostridium difficile–associated diarrhea. While there are some similarities in opinions between adult and pediatric intensivists, there are also some differences with respect to a trial of stress ulcer prophylaxis. For example, pediatric intensivists in the current study placed less emphasis than adult intensivists in the previous study10 on invasive mechanical ventilation as an indication for stress ulcer prophylaxis (7% versus 60%). The reasons for this difference are unclear. Similarly, 76% of adult intensivists reported stopping stress ulcer prophylaxis in practice when patients were no longer mechanically ventilated, compared to 18% of pediatric intensivists. Of particular importance is the role of enteral feeding, which appears to be more important for pediatric intensivists. These differences suggest that inclusion criteria and duration of therapy should be considered differently in adult and pediatric trials.
The strengths of this survey study included the strategy that we used to identify all potential respondents, the reasonably high response rate (similar to the median response rate of 63% reported in surveys of critical care clinicians12), and representation from all of the PICUs in Canada. This survey study also had some limitations. We did not formally test the psychometric properties of the questionnaire. We sent the survey on behalf of an influential critical care trials group, which may have led some respondents to endorse more positive views of a trial because of social desirability bias. Finally, physicians in other countries may have different views and attitudes toward a future trial because of differences in patient populations, in particular the baseline risk of bleeding, or differences in clinical practice and attitudes toward research.
If a future RCT of stress ulcer prophylaxis is to be successful, it must be acceptable to clinicians, researchers, and parents. We anticipate that their views are likely to be highly context dependent, depending on issues such as details of the protocol and available trial resources. To reduce bias among respondents in the current study, we did not mention the details of a specific trial; however, responses might have been different if we had specified that we are planning a placebo-controlled, non-inferiority RCT. We will use the results of this survey, along with a published survey of stated practice patterns,13 an ongoing observational study of actual clinical practice, and an ongoing pilot RCT (NCT02929563),14 to inform discussions with clinicians, researchers, and parents, with the goal of achieving consensus. The attitudes of clinicians in other countries will also be important, as a future trial will likely need to be a multinational effort.
This survey study showed clear support for a placebo-controlled RCT of stress ulcer prophylaxis among pediatric critical care physicians in Canada. We are using the results of this survey to help build consensus on key elements of the trial design in the Canadian research and clinical pediatric critical care community.
1 Costarino AT, Dai D, Feng R, Feudtner C, Guevara JP. Gastric acid suppressant prophylaxis in pediatric intensive care: current practice as reflected in a large administrative database. Pediatr Crit Care Med. 2015;16(7):605–12.
2 Araujo TE, Vieira SMG, Carvalho PRA. Stress ulcer prophylaxis in pediatric intensive care units = Profilaxia para úlcera de estresse em pacientes internados em UTI pediátrica. J Pediatr (Rio J). 2010;86(6):525–30. Article in Portuguese, English abstract.
3 Lacroix J, Infante-Rivard C, Gauthier M, Rousseau E, van Doesburg N. Upper gastrointestinal tract bleeding acquired in a pediatric intensive care unit: prophylaxis trial with cimetidine. J Pediatr. 1986;108(6):1015–8.
4 López-Herce J, Dorao P, Elola P, Delgado MA, Ruza F, Madero R. Frequency and prophylaxis of upper gastrointestinal hemorrhage in critically ill children: a prospective study comparing the efficacy of almagate, ranitidine, and sucralfate. Crit Care Med. 1992;20(8):1082–9.
5 Yildizdas D, Yapicioglu H, Yilmaz HL. Occurrence of ventilator-associated pneumonia in mechanically ventilated pediatric intensive care patients during stress ulcer prophylaxis with sucralfate, ranitidine, and omeprazole. J Crit Care. 2002;17(4):240–5.
6 Duffett M, Choong K, Foster J, Gilfoyle E, Lacroix J, Randolph A, et al. Stress ulcer prophylaxis in critically ill children: a systematic review [abstract PICC-0055]. In: 8th World Congress on Pediatric Intensive and Critical Care; 2016 June 4–8; Toronto (ON). Available from: www.picc2016.com/PublishingImages/scientific-information-abstracts/scientific-program/PICC%202016%20posters%20abstracts%20all.pdf
7 Albert BD, Zurakowski D, Bechard LJ, Priebe GP, Duggan CP, Heyland DK, et al. Enteral nutrition and acid-suppressive therapy in the PICU: impact on the risk of ventilator-associated pneumonia. Pediatr Crit Care Med. 2016; 17(10):924–9.

8 Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007: 102(9):2047–56.
9 Duffett M, Choong K, Hartling L, Menon K, Thabane L, Cook DJ. Randomized controlled trials in pediatric critical care: a scoping review. Crit Care. 2013;17(5):R256.

10 Shears M, Alhazzani W, Marshall JC, Muscedere J, Hall R, English SW, et al. Stress ulcer prophylaxis in critical illness: a Canadian survey. Can J Anaesth. 2016;63(6):718–24.
11 Duffett M, Choong K, Vanniyasingam T, Thabane L, Cook DJ. Making decisions about medications in critically ill children: a survey of Canadian pediatric critical care clinicians. Pediatr Crit Care Med. 2015;16(1):21–8.
12 Duffett M, Burns KE, Adhikari NK, Arnold DM, Lauzier F, Kho ME, et al. Quality of reporting of surveys in critical care journals: a methodologic review. Crit Care Med. 2012;40(2):441–9.
13 Ouellet J, Bailey D, Samson MÈ. Current opinions on stress-related mucosal disease prevention in Canadian pediatric intensive care units. J Pediatr Pharmacol Ther. 2015;20(4):299–308.

14 McMaster University. Pediatric intensive care ulcer prophylaxis pilot trial (PIC-UP) [trial registration]. ClinicalTrials.gov identifier NCT02929563. Bethesda (MD): National Institutes of Health; 2016 Oct 3 [updated 2017 May 15; cited 2017 Jul 4]. Available from: https://clinicaltrials.gov/ct2/show/NCT02929563
Competing interests: None declared. ( Return to Text )
Mark Duffett is the author of the “Con” side of this issue’s Point Counter-point debate concerning stress ulcer prophylaxis; see page 317. ( Return to Text )
Funding: This study was funded by a Hamilton Health Sciences Clinical Health Professional Award. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 70, NUMBER 4, July-August 2017