Piera Polidori, Carlo Cifani, Carlo Polidori
The central body of the Italian National Healthcare Service (NHS) is the Ministry of Health, which coordinates the NHS and is responsible for government functions related to health care for Italy’s 60 million inhabitants.
The Ministry of Health works in collaboration with the governments of the 20 Italian regions, 15 of which operate under a “normal” statute and 5 under a “special” statute. The NHS develops a National Healthcare Plan (Piano Sanitario Nazionale), proposed by the Ministry of Health, which then is put into effect by the government, keeping in mind the proposals made by the individual regions. This 3-year plan indicates the priority areas where work is needed to reduce social inequalities in health care. It is used to define general objectives and methods for carrying out the institutional activities of the NHS. The National Healthcare Plan stipulates various essential levels of assistance (Livelli Essenziali di Assistenza or LEAs), which represent guarantees of the objective of social equality among all citizens and which are necessary to meet the fundamental needs of promotion, maintenance, and recovery of health; the Plan also establishes the modalities for meeting these goals. The LEAs are the activities and services that the NHS is bound to guarantee for all citizens, free of charge or with copayment, by collecting funds through the taxation system. At the regional level, the regions follow a 3-year Regional Healthcare Plan (Piano Sanitario Regionale) for putting the NHS plan into action, taking into consideration both national and regional objectives.
The Italian NHS offers universal assistance, that is, medical treatment to all those who need it. To guarantee the sustainability of such a system, considering the increasing cost of innovative medicines and medical devices, all of the health care professionals involved must take great care to monitor the appropriateness of diagnostic and treatment plans to ensure that the measures taken correspond to patients’ needs, according to the correct instructions for medicines, in terms of efficacy for a specific pathology; these measures must be provided in suitable ways and within adequate times, on the basis of recognized standards, with a positive balance between benefits, risks, and costs.1
The NHS carries out its functions through the 20 regional governments, by means of hospital firms (Aziende Ospedaliere) and health care firms (Aziende Sanitarie), each health care firm being an administrative aggregation of more than 2 hospitals. Through these hospital and health care firms, the NHS provides most of the health care assistance in Italy, with the remainder being provided by private structures that operate through an agreement with the national system.
In this context, the NHS pharmacists play a crucial role, inasmuch as Italy has 2 distinct, but well-integrated types of pharmacist, the first working in hospital pharmacies and the second, known as territorial pharmacists, working in the pharmaceutical services of the local or provincial health care firms. These 2 types of pharmacists work together with other health care professionals as members of treatment commissions or multidisciplinary work groups to evaluate the use of medicines according to appropriateness criteria based on current legislation, scientific evidence, and economic evaluations.
An overview of the total number of pharmacists and the workload for hospital pharmacists appears in Table 1.2
Table 1 Number of Pharmacists in Italy and Number of Beds Served by Each Hospital Pharmacist*
Hospital pharmacists also formulate shared Diagnostic and Treatment Itineraries of Assistance. For this, they consider not only medicines but also clinical practice, examining all aspects of the pharmacological treatment of individual patients and taking into account the patients’ specific and particular characteristics. As such, these pharmacists contribute to the personalization of treatment and support specialists in optimization of that treatment.
In addition to the 2 types of pharmacist described above, Italy also has community pharmacists, whose important contributing role is not part of the NHS, but who work through an agreement with the NHS. Their total number in Italy is about 50 000, and they work in community pharmacies that are open to all citizens. They distribute prescription and over-the-counter drugs and prepare compounded drugs, working under a for-profit model. In addition, they are involved in pharmacovigilance, health-related education, and health promotion.
The NHS pharmacists guarantee the quality of pharmaceutical assistance and the correct balance between appropriateness, quality, and costs, especially through continual monitoring of the appropriate use of drugs and medical devices.
In the United States and some European countries, hospital pharmacists have for years carried out much of their activity in hospital wards, alongside physicians and nurses, on increasingly specialized and multidisciplinary teams.
In Italy, some experiences of this kind have been described, especially in oncology and antimicrobial stewardship, but the model of the hospital ward or department pharmacist has yet to become widespread. Conversely, hospital pharmacists intervene on a daily basis in assessing the appropriateness of prescriptions, assessing the quality of pharmaceutical assistance, and controlling expenditures, in full collaboration with the other operative units and services involved in the process of pharmaceutical therapy in the hospital setting.3–5
Hospital pharmacists are active on numerous commissions of their health care firms, for example, those concerned with infections, treatments, or health technology assessment. In this capacity, they help in making decisions about the appropriate use of medicines and medical devices, and in choosing which medicines and medical devices should be purchased according to criteria of appropriateness, in line with current legislation and guidelines.6
In addition, in dispensing medications for patients who are in the first cycle of treatment (called “direct distribution” [distribuzione diretta]) or for those who travel from one Italian region to another for treatment, hospital pharmacists are particularly active in guaranteeing appropriateness and adherence with current legislation. In this context, they provide information to patients for home treatment, underlining the importance of compliance with the physician’s prescriptions and instructions. The hospital pharmacist is also involved in medication reconciliation when patients are admitted, during their hospital stay, and when they are discharged. For example, in the main hospital in the Marche Region, which has 1000 beds, hospital pharmacists examine pharmacological treatment plans for patients, and every month propose modifications for an average of 600 drug treatment plans, most of which are related to cancer and cardiology treatments; at this hospital, each treatment plan is managed by 2 hospital pharmacists.
Another important role for hospital pharmacists is serving as members of the Ethics Commission of their respective health care firms. In this role, they often run the scientific secretariat of the Commission and serve in the office that reports on clinical trials conducted at the hospital. In addition, hospital pharmacists are increasingly active in clinical trials, sometimes as principal investigators. Hospital pharmacists are also responsible for managing experimental medicines during clinical trials. In the case of parenteral administration, it is the hospital pharmacist’s duty to prepare the experimental drugs for administration.
In some health care firms, the hospital pharmacies have hazardous drug compounding laboratories where anticancer drugs are diluted into infusion bags. In these firms, this important service is provided by hospital pharmacists, who follow national guidelines developed by the Italian Society of Hospital and Territorial Pharmacists (Società Italiana di Farmacia Ospedaliera e dei Servizi Farmaceutici delle Aziende Sanitarie, known as SIFO) to ensure the quality of compounded drugs. For example, in the main hospital of the Marche Region, 3 full-time pharmacists prepare, with the help of a robot, about 25 000 oncology infusion bags a year. Other duties of the hospital pharmacist are the formulation or set-up of personalized preparations for individual patients, including bags for total parenteral nutrition, specific arrangements for drugs not available on the market and required for treatment of rare diseases, and preparation of unit doses of drugs. A 2005 study7 reported that hospital pharmacists in the 9 major Italian hospitals for pediatric patients made ready about 100 000 personalized preparations for pediatric patients or premature newborns. Finally, hospital pharmacists are involved in quality control of the radiopharmaceuticals used in the hospital.
In the sphere of oncology, hospital pharmacists are responsible for writing, codifying, and updating treatment protocols proposed by clinicians, checking to ensure that they correspond to the criteria of scientific and legislative appropriateness. In addition, for each individual patient, hospital pharmacists check that the approved treatment plans are properly applied, for example, in terms of instructions for use, dosage, posology, infusion time, and dilution solvents. Hospital pharmacists are also responsible for ensuring quality during set-up and administration, by analyzing processes and writing procedures and instructions to reduce risk. Because examination of the appropriateness of prescriptions is specifically a duty of hospital pharmacists, these health care providers have been integrated into the Italian Medicine Agency (Agenzia Italiana del Farmaco) system for drug monitoring via online registers. In general, they monitor the use of all drugs administered in the hospital, in terms of consumption and expense, as well as in terms of the individual patient (e.g., if drugs are to be administered in a particular way for treatment of a given patient), with specific attention to indicators of appropriateness detailed at the regional or national level. The number of times that the online register is accessed varies with the number of beds in each hospital. For example, in a hospital of 300 beds, the register was accessed on average 30 times a month to request reimbursement of hospital expenses incurred in dispensing oncology drugs and about 300 times a month to register the unit doses of special drugs delivered.
Hospital pharmacists engage in intense activity of formation (producing evidence about drug-related activity and medical device efficiency) and information (collecting data) about medicines and medical devices. Some hospitals have a formal centre that supports physicians and nurses with information about drugs, for example, regarding dosages, indications, contraindications, or any particular information requested.
Finally, hospital pharmacists make an important contribution to pharmacovigilance and medical device vigilance, monitoring of drug interactions, and management of clinical risk. For years, national and regional health care policies have sought to reduce clinical risk, especially in the management of drugs, as demonstrated by the publication of recommendation 7 of the Ministry of Health (“Recommendations for the prevention of death, coma or grave harm caused by errors in pharmaceutical treatment: an incorrect use of drugs can cause adverse events with grave consequences for the patient”) and by subsequent recommendations 12, 14, and 17.8 This is surely a sphere in which ongoing work must be done to optimize strategies for reducing and preventing errors.8
One other important function of the hospital pharmacist is to develop the hospital budget for drugs and medical devices and to ensure that the hospital adheres to it.
Finally, in some health care firms, hospital pharmacists are involved in the process of obtaining Ministry of Health quality accreditation for the daily activities of the hospital pharmacy. This is accomplished by reporting that all of the technological and organizational processes required by the International Joint Commission or by the International Organization for Standardization are present in the hospital’s pharmacy.
The second type of pharmacist involved in hospital work, with the same employment contract as a hospital pharmacist but having different activities, is the territorial pharmacist (a role that is distinct from that of the community pharmacist). To our knowledge, the territorial pharmacist is exclusive to the Italian NHS, with no counterpart in other European Union countries, the United States, or other nations. Like the hospital pharmacist, the territorial pharmacist plays a key role within Italian local health authorities in enhancing the appropriateness of prescriptions. The territorial pharmacist distributes specific drugs that are prescribed by hospital physicians but are not available in the community pharmacy, such as growth hormones, antiemetics, and monoclonal antibodies, at selling prices determined by the hospital. This distribution is called “direct distribution” (distribuzione diretta), and the service takes place in the pharmacy of the hospital or in a different building. The drugs distributed by the territorial pharmacist are under the strict control of the Italian Medicine Agency. During the period January to August 2016, total direct distribution sales in Italy were about 3.7 billion euros for class A drugs (essential drugs for chronic conditions, which are distributed by the NHS at no cost to patients). Several studies conducted by hospital pharmacists have demonstrated that the expertise of hospital and territorial pharmacists reduced the total cost of drugs that the NHS provides to the Italian population by about 20%.9
Another activity of territorial pharmacists is the provision of pharmaceutical service’s, which involves examining prescription data from community pharmacies that have agreements with the NHS, through which community pharmacists distribute drugs for the hospital at selling prices determined by the hospital. This drug management system is called “distribution on behalf of the local health care firm” (distribuzione per conto). For example, for the year 2016 in the Marche Region (population 307 000), 3 million filled prescriptions were checked by 4 full-time territorial pharmacists. For the most part, the data collected are related to patients with diabetes, arterial hypertension, osteoporosis, or other chronic conditions. One difficulty that territorial pharmacists encounter in examining prescription data from community pharmacies is that the reports do not indicate the diagnosis, except in cases where the patient has taken measures to obtain an exemption from copayment because of an ongoing condition; in that situation, the physician indicates the “exemption due to pathology” code on the prescription. This lack of information about the diagnosis makes it difficult to ascertain whether the dosage, posology, and length of treatment are appropriate.
In an effort to optimize appropriateness of prescriptions, territorial pharmacists send reports to general practitioners (Medico di Medicina Generale) and to pediatricians (Pediatra di Libera Scelta) comparing the number of their prescriptions with those of other professionals regionally and nationally. They also organize meetings to address problems such as overprescribing and to improve the services provided by these 2 types of health care professionals.
Territorial pharmacists may also serve as members of the boards of directors of the local health care firms or as representatives to medical organizations, analyzing reports on pharmaceutical prescriptions in their specific districts. These reports detail gross per capita expenditure on class A drugs (which are paid entirely by the Italian NHS) that have been dispensed by community pharmacies and highlight how these expenditures differ from regional and national averages for total consumption, according to the Anatomical Therapeutic Chemical (ATC) classification of drugs, in terms of defined daily dose (DDD) per 1000 inhabitants and expenditure per 1000 inhabitants.
Another set of parameters analyzed by the board of each local health care firm are the expenditure and DDD for generic drugs relative to the total number of prescriptions. The board monitors the quality of the drugs (according to ATC classification, brand name, generic name, etc.) and the quantity (posology, length of treatment, and compliance with treatment), with attention to spending limits for each ATC class, in cases where these limits have been established by regional legislative decrees.
On a regular basis, the board analyzes the reports, identifies problem areas, and decides what actions to take and for which categories of drugs. Each health care firm may choose the actions it deems most useful, for example, promoting the presence of generic drugs on the market (when existing use of generic products is too limited) or focusing on categories of drugs with consumption that exceeds regional and national averages in terms of DDD per 1000 inhabitants or average per capita expenditure.
Once the board has examined the prescription reports, it works to inform and educate physicians to resolve the problems that have emerged. Specific meetings and courses are organized in the districts, during which the territorial pharmacist describes the consumption data for the drug class in question, explains the approved instructions for use, illustrates cost comparisons for the various active ingredients, and communicates the problem areas identified during monitoring, including treatment switches and compliance with treatment instructions. In most districts in Italy, these meetings take place 6 times a year. The general practitioners and/or pediatricians at each gathering bring their experience to bear on the discussion by presenting clinical cases. Starting from the diagnosis of a given condition, the guidelines and protocols for diagnosis and treatment are examined and the efficacy of the drug is discussed, particularly whether it is effective in terms of clinical end points (i.e., not just in the laboratory). There is also in-depth discussion of cases in which the patient presents with factors that could heighten or reduce the drug’s effect or that predispose the patient to adverse events.6 The discussion also covers the correct dose, whether the posology prescribed is simple for the patient to follow, the instructions that should be given to facilitate consumption of the drug, potential interactions with other drug categories or with foods, management of adverse events, the appropriate duration of treatment for each condition, and finally which treatment costs the least, with equivalent effectiveness.
Territorial pharmacists are indispensable for the monitoring and analysis of treatments, bringing their specialized knowledge and skills to these meetings and courses. They also prepare a final document for the physicians to explore aspects of treating a given condition and the appropriate use of drugs. Their activities ensure that the information provided is independent and correct, which may not always be the case when physicians rely exclusively on explanations provided by pharmaceutical firms.
In the case of some categories of drugs that patients often buy without a prescription, the board may decide to organize campaigns to educate the public about indications, contraindications, and side effects in the case of inappropriate use. The territorial pharmacist then prepares pamphlets to distribute to the public and educational posters for doctors’ waiting rooms.
Following these activities, the board may evaluate the results achieved in terms of appropriateness of prescriptions by comparing clinical data against predefined standards, such as prescriptions exceeding regional and national averages, prescriptions for drugs not considered to be the first choice for a particular condition, and percentage of generic drugs out of the total number of prescriptions for a given category. Wherever this model has been applied, the results have been increased awareness among physicians and reduced expenditures for drugs.
Another important service provided by territorial pharmacists involves reconciliation of prescriptions and treatments for patients who transition from one care setting to another, for example, patients discharged from a hospital ward who go on to an assisted health care residence or patients with a terminal illness who leave their homes for hospice. Many of these patients are taking a number of drugs, and it is important to be alert to potential drug interactions and/or adverse drug reactions. Considerable attention must be devoted to delivery of medical devices for patients who have undergone gastrostomy, urostomy, or tracheotomy; patients with bedsores; and those who have a catheter. In these cases as well, monitoring is required to ensure that the device is appropriate for the patient’s condition and that there is compliance by the patient or caregiver.
Finally, territorial pharmacists inspect community pharmacies to ensure they comply with Italian laws regulating organization and technical equipment, as well as those regarding the management and disposal of narcotics.
In the mid-1990s, Italian universities established schools of specialization in the pharmaceutical field, called “schools of specialization in hospital pharmacy”. Since then, to attain access to such a school, students must first complete a 5-year pharmacy program in any Italian or foreign university. Then, after passing the board certification test, they must pass another selection test (in pharmacology, pharmaceutical chemistry, and compounding) at a university where this postgraduate program is offered. Indeed, not all schools of pharmacy have started the program, and today only 21 of the 24 Italian schools of pharmacy have a program of specialization in hospital pharmacy (and of these, not all are currently running).
In response to innovations that pharmaceutical firms have been implementing with hospitals to improve the quality of patient care and changes in the pharmacist’s role in this setting, pharmacy faculty members realized that a typical 5-year course of pharmacy was no longer sufficient preparation for work in hospital pharmacies, and decided that at least 2 further years of specialization were needed. A few years later, in 1995, Italian universities reached an agreement that the academic program for such a “school of specialization in hospital pharmacy” should last at least 3 years. This process of recognizing the need to expand training to encompass new issues emerged through contact between experienced pharmacists who had been working in hospital settings for several years and professors teaching in faculties of pharmacy. This new degree program of specialization has led to a variety of new courses, such as pharmacokinetics, radiotherapy, and hospital and health organization in Italy. Practice in the hospital setting became a mandatory part of the program. During their hospital internship, students gain practical experience with such tasks as preparing bags for anticancer drug infusions, defining diets for underweight newborns, and other administrative duties, including ordering drugs from manufacturers. The time devoted to this practical work at the hospital pharmacy was set at about 500 hours per year.
In the meantime, through law 509, the Italian Ministry for Education and Research introduced the concept of “credits” in November 1999, defining a credit as 25 hours of study for a university student, and specifying that a student must earn 60 credits each year. Therefore, to earn a bachelor degree in pharmacy, a student had to obtain 300 credits over 5 years of education. This concept of credits was then extended to the higher education program of specialization. Indeed, the 2005 law defined for each area the maximum number of credits required to prepare a student in the field of hospital pharmacy. For example, the core number of credits for this specialization was 195 out of the 300 total credits, including pharmacology and pharmaceutical compounding.
The 2005 law concerned the reorganization of all kinds of schools of specialization in the health system, including the hospital pharmacy degree program, adding an academic year to the previous 3-year program. This new 4-year program introduced classes on pharmacovigilance, hospital bioethics, health technology assessment, and Italian legislation for the approval of medical devices.
The most impressive development arising from the new law in 2005 was the change in the number of students allowed to attend these programs, calculated in relation to the turnover of hospital pharmacists in the Italian health system. The total number of students was set at 150 for the entire country, with an average of 8 students per school being eligible. Thus the only criterion was the need to replace those who retired or changed fields, to maintain equilibrium in the health care system.
An important factor was introduced with a new law in 2006, concerning the general organization of schools of specialization in the health system. Each school had to demonstrate to the government that its students did their practical work in multiple hospitals. To this end, the Italian Ministry of Education and Research organized an online national database where the directors of the schools of specialization in hospital pharmacy could record the names of the hospitals involved in student training for their respective programs.
As mentioned above, the teaching program for specialization in hospital pharmacy, based upon the 2005 law, is organized into 4 academic years, with 30% of the total credits earned through courses and the remaining 70% through hospital training within the school’s network.
For the 30% of the program devoted to coursework at the university, the program contains 4 or 5 courses per year. Student evaluations have indicated that the first-year courses in pathology and biostatistics are the most appreciated, whereas in the second year pharmacokinetics and nutrition courses receive positive feedback. In the third year, students have found most interesting their courses in radiopharmacy and clinical pharmacology. In the final year, courses about clinical pharmacology and management of the hospital setting have been viewed as the most innovative.
For the 70% of the program based on in-hospital training, the Italian Society of Hospital and Territorial Pharmacists prepared a document that was distributed to all universities with the degree program in hospital pharmacy and to all hospital pharmacy directors of each school’s hospital network. This document was essentially a copy of the pertinent law concerning practical training, but it also detailed all of the practical skills that students should master during their training, indicating the order in which these skills should be developed throughout the 4 years of the program.
The law of 2015 (Decreto Interministeriale 68, dated February 4, 2015) makes it clear that students must do more hours of training. It is to be hoped that through this additional training time, the students will gain more clinical experience. In most European countries, hospital pharmacists do rounds with clinicians, suggesting and in some cases prescribing, together with the physician, the best drug for each patient, at the patient’s bedside. In Italy, however, clinical pharmacists have had little experience of this type in recent years; when such experience has occurred, it has been prompted primarily by a physician’s simple request for the hospital pharmacist’s collaboration. Given their extensive knowledge about drugs, hospital pharmacists should be collaborating with clinicians, drawing upon scientific data and evidence-based medicine data. In this context, the hospital pharmacist could give the physician great help in managing risks due to drug use in the clinical setting. In fact, the Italian NHS has just published a recommendation on how the hospital pharmacist and other health care professionals should develop processes for safety in health care to reduce therapeutic errors.
Since the mid-1990s, efforts to develop better schools of specialization have been ongoing. Credit for this improvement is surely due to the strong interaction between scientific societies and universities. Three main things must merge harmoniously to promote better-quality health care and harmonious growth of the system: people, knowledge, and resources.
The organization of the Italian NHS is complex, but the system is founded on principles of universality and equality. Monitoring the appropriateness of prescriptions is a valid approach for all regions of the country, but above all for those that are under a specific management regime to bring expenditures back to budgeted levels and to govern the efficacy, efficiency, and costs of drugs and health care in general.
The training and skills of hospital pharmacists and territorial pharmacists, which include not only specialist skills in pharmacology, but also managerial abilities, are indispensable for optimizing the decision-making processes for selection and prescription of therapeutic drugs, diagnostic drugs, and medical devices.
It is evident that pharmacists carry out their activities with constant attention to the health needs of citizens, by educating patients about the correct use of drugs that are prescribed and by ensuring the quality, efficacy, and safety of the products prepared and distributed, without neglecting the pharmacological and cost aspects related to their use.
For this reason, it is fundamental that university undergraduate and graduate programs continually update their offerings, with active contributions from the country’s scientific community, especially the Italian Society of Hospital and Territorial Pharmacists, to meet the ever-growing and changing needs dictated by daily work in hospital and territorial pharmaceutical structures.
1 Manuale di formazione per il governo clinico: appropriatezza [Clinical governance training manual: appropriateness]. Rome (Italy): Ministero della Salute; 2012 [cited 2016 Jun 20]. Available from: www.salute.gov.it/imgs/C_17_pubblicazioni_1826_allegato.pdf. In Italian.
2 Personale delle A.S.L. e degli Istituti di ricovero pubblici ed equiparati Anno 2013 [Staff and other personnel working at public and private hospital firms for the year 2013]. Rome (Italy): Ministero della Salute, Direzione Generale della Digitalizzazione, del Sistema Informativo Sanitario e delle Statistica, Ufficio di Statistica; [cited 2017 Jul 25]. Available from: www.salute.gov.it/imgs/C_17_pubblicazioni_2605_allegato.pdf. In Italian.
3 Carollo A. Italy. In: The role of the pharmacist in a multidisciplinary team. Hosp Pharm Eur. 2014 [cited 2017 Jul 21];(73). Available from: www.hospitalpharmacyeurope.com/featured-articles/role-pharmacist-multidisciplinary-team
4 O’Connor MN, Gallagher P, O’Mahony D. Inappropriate prescribing: criteria, detection and prevention. Drugs Aging. 2012;29(6):437–52.
5 Barber N, Bradley C, Barry C, Stevenson F, Britten N, Jenkins L. Measuring the appropriateness of prescribing in primary care: are current measures complete? J Clin Pharm Ther. 2005;30(6):533–9.
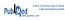
6 Appropriatezza nel trattamento dell’osteoporosi. AIFA presenta il nuovo algoritmo [Appropriateness in the treatment of osteoporosis: AIFA presents new algorithm]. Rome (Italy): Agenzia Italiana del Farmaco; 2016 [cited 2016 Jun 20]. Available from: www.aifa.gov.it/it/content/appropriatezza-nel-trattamentodell%E2%80%99osteoporosi-aifa-presenta-il-nuovo-algoritmo. In Italian.
7 Associazione Ospedali Pediatrici Italiani. L’allestimento di preparati farmaceutici personalizzati nelle UUOO di farmacia degli ospedali pediatrici italiani. Boll SIFO. 2005;51(5):212–8. In Italian.
8 Raccomandazioni agli operatori [Recommendations to the operators]. Rome (Italy): Ministero della Salute; 2007 [updated 2015 Apr 23; cited 2016 Jun 20]. Available from: www.salute.gov.it/portale/temi/p2_6.jsp?id=250&area=qualita&menu=sicurezza. In Italian.
9 Congedo R, Cogo M. Erogazione della terapia in dimissione: un servizio o un risparmio? Boll SIFO. 2003;49(5):270–5. In Italian.
Competing interests: None declared. ( Return to Text )
Acknowledgement: The authors thank Sheila Beatty for translation of this article from Italian to English.
Canadian Journal of Hospital Pharmacy, VOLUME 70, NUMBER 4, July-August 2017