Dorothy Aywak, Collins D P Jaguga, Nancy G Nkonge, Rosaline Kinuthia, Claris Ambale, Ismail A Awle
Kenya is located in Eastern Africa, on the coast of the Indian Ocean. The country is bordered by Tanzania, Uganda, Ethiopia, South Sudan, and Somalia. It encompasses savannah, lake lands, the Great Rift Valley, and mountain highlands, with abundant wildlife and a human population of about 47.6 million.1 With a gross domestic product (GDP) of US$1143 per capita in 2016, Kenya is considered a lower-middle income country.2
In this paper, we discuss pharmacy practice in Kenya in terms of the World Health Organization health system building blocks: health system leadership and governance; health care financing; health information and research; health workforce, health service delivery; and access to medical products, vaccines, and technology. The paper concludes with information about the future direction of pharmacy practice in Kenya.
Over the past 2 decades, there has been improvement in Kenya’s health profile.3 Life expectancy at birth has improved, from 60 years in 2009 to 61 years in 2013. However, the adult mortality rate (for those 15–60 years of age) has increased from 258 deaths per 1000 in 1990 to 274 deaths per 1000 in 2013, possibly because of noncommunicable diseases and injuries. Maternal mortality rate has declined from 490 deaths per 100 000 live births in 1990 to 106 deaths per 100 000 live births in 2015/16, with 27 of the 47 counties having a rate below 100 deaths per 100 000 births (see below for information about Kenya’s counties). The under-5 mortality rate (in terms of deaths per 1000 live births) declined from 296 in 1990 to 49 in 2015. The infant mortality rate (from birth to age 1 year; in terms of deaths per 1000 live births) decreased from 64 in 1990 to 36 in 2015.3
These gains can be attributed to the development and implementation of the Kenya Health Policy Framework (1994–2010), the government’s Vision 2030, promulgation of the Kenya Constitution of 2010,4 and fast-tracking of actions to achieve the Millennium Development Goals by the year 2015.5 All of these initiatives have greatly influenced the health status of Kenyans and the structure within which health services are provided. In particular, the new constitution has created a devolved system of governance with 47 counties, each of which is responsible for providing and delivering health care services to its citizens. The devolved system is intended to make the right to health a reality for all Kenyans.
Health care in Kenya is overseen by governments at the national and county levels, which have functions that are distinct yet interdependent. The national government is responsible for leadership in health policy development; management of national referral health facilities; capacity-building and provision of technical assistance to counties; and consumer protection, including the development of norms, standards, and guidelines. The county governments are responsible for health services, including county health facilities and pharmacies, ambulance services, promotion of primary health care, licensing and control of food retail outlets, and solid waste disposal.5
The most recent Kenya National Health Accounts survey was undertaken in 2015 to track the flow of funds in the health sector for the year 2012/13.6 Total health expenditures in that year were US$2743 million, up from US$2155 million in 2009/10. Total health spending in 2012/13 accounted for 6.8% of GDP, up from 5.4% in 2009/10. The government’s expenditures on health as a percentage of total expenditures increased from 4.6% in 2009/10 to 6.1% in 2012/13, with per capita expenditure increasing from US$56 in 2009/10 to US$67 in 2012/13.6 The private sector continues to be the major source of health care financing, contributing 40% of total health expenditures in 2012/13, up from 37% in 2009/10. The government’s contribution to total health expenditure was 34% in 2012/13, an increase of 17 percentage points over the 2009/10 estimates. Donor contributions (from foreign countries and international nongovernmental organizations) accounted for 26% of total health expenditure in 2012/13, down from 35% in 2009/10. This is the first time that donor funding for the health sector has declined.6
The District Health Information System (version 2) is used in the public health system for the reporting of health indicators.7 It is used in combination with manual medical records because only 10% of public hospitals (mostly the national and county referral hospitals) have electronic medical records.8 In contrast, most of the private hospitals have electronic systems for patient records and medication supply. The quality of data in the District Health Information System is limited by constraints such as inadequate financial and human resources, limited quality assurance, and minimal supervisory support.9
Public hospitals that procure medicines and medical products from the Kenya Medical Supplies Authority (KEMSA), a government entity, must use the standard KEMSA ordering form, which is electronic. Consumption reports for antiretroviral medication, antitubercular medication, and contraceptives are conveyed electronically to the respective programs from the facilities. The Pharmacy and Poisons Board (PPB) has a pharmacovigilance reporting system with an electronic platform that allows for real-time reporting of adverse drug reactions and poor-quality medicine.10
The Kenya Medical Research Institute is the government body mandated with health care research. The 2 national referral hospitals also conduct health care research in collaboration with universities.11–13
In Kenya, there are 24 separate groups of health care personnel regulated by the government, encompassing both clinical and nonclinical personnel. The clinical groups include medical practitioners, pharmacists, nurses, dentists, nutritionists, physiotherapists, and laboratory technologists.14 Traditional and alternative medicine practitioners are considered alternative health care providers, who are regulated through the Ministry of Gender and Culture; however, efforts are under way to have their practice regulated by the Ministry of Health. A system of validation of herbal practitioners is ongoing through the University of Nairobi School of Pharmacy (Department of Pharmacognosy and Pharmacology). Traditional practitioners are another option for alternative health care; however, the concept of complementary medicine is slowly being accepted among conventional practitioners as well. One of the referral hospitals, Kenyatta National Hospital, has piloted a complementary medicine service to address the unmet needs of comprehensive medication review. Conventional medicine practitioners have been sensitized to the role that complementary medicine plays, especially in the management of patients with chronic illnesses. The government recently recognized the role they play and following gazettement of the Health Act in 2017, the Ministry of Health has been directed to develop guidelines to facilitate cross-referral between conventional and traditional health care practitioners. The Health Act also directs Parliament to establish a regulatory body to manage the practice of traditional and alternative medicine.15
To be registered as a pharmacist in Kenya, one must complete a Bachelor of Pharmacy degree program approved by the Commission of University Education, followed by a 1-year supervised internship, regulated by the PPB. Those who undertake the degree program outside the country are required to sit a pre-internship examination. The internship involves 6 months of hospital pharmacy–based practice, 3 months in a community (retail) pharmacy, and 3 months in the pharmaceutical industry. Upon completion of their internship, practitioners undertake a registration examination administered by the PPB.16 The Pharmaceutical Society of Kenya is the professional body that promotes common standards for professional conduct and a code of ethics, as well as being an advocate for the welfare of pharmacists. All pharmacists are encouraged to participate in continuing professional development activities, to continuously update their knowledge and skills in order to provide safe and effective pharmaceutical care to patients.17
The first set of Kenya-trained pharmacists graduated in 1978, from a 4-year program in a single public university. Since then, the number of institutions offering pharmacy degrees has increased to 7 universities, both public and private. Pharmacy education in these institutions is accredited by both the Commission of University Education and the PPB. The full-time degree program has now increased to 5 years in duration.16
Pharmacists are supported in service delivery by pharmaceutical technologists (also referred to as pharmacy technicians in other countries), who are also regulated by the PPB. In the hospital setting, the pharmaceutical technologists support pharmacists by performing tasks such as inventory management and dispensing. In the community setting, pharmaceutical technologists often practise without direct supervision, running dispensing outlets after meeting the PPB’s regulatory requirements. A total of 24 non-university higher education institutions are accredited by the PPB to offer a diploma in pharmaceutical technology.18
Traditionally, professional practice in pharmacy has been oriented toward product manufacture and supply; however, in parallel with international trends, there has been a paradigm shift toward more cognitive patient-oriented practice. The Purdue Kenya Partnership, which involves the Purdue University School of Pharmacy and Pharmaceutical Sciences (in West Lafayette, Indiana, USA) and the Moi University School of Medicine and the Moi Teaching and Referral Hospital (both in Eldoret, Kenya), is a unique collaboration that offers residency training in clinical pharmacy. This residency program pairs postgraduate pharmacy trainees from the 2 countries to collaboratively build patient care and leadership skills. The program’s success has been lauded, and the Kenyan government is considering formalizing it into a master’s level program.16,19
With the devolution of health care to the 47 counties, the county hospitals have begun offering specialized services such as renal dialysis and critical care. These new services have, in turn, created the need to build capacity and enhance the skills of the health care providers working in these hospitals, including pharmacists. In response, the East African Kidney Institute of the University of Nairobi, in collaboration with Kenyatta National Hospital, now provides a 3-month residency training program on the management of renal and urological diseases.20 The first cohort of 36 health care providers included 5 pharmacists under the preceptorship of pharmacists at Kenyatta National Hospital and University of Nairobi on pharmacotherapy in renal disease. The program involves multi-disciplinary teaching sessions and ward rounds mainly in the renal unit. The pharmacy residents are exposed to pharmacy procedures that support successful transplants and post-transplant care.
Despite these opportunities, many pharmacists seek pharmacy residency training abroad to gain competencies in clinically oriented practice, as this approach is not yet universal in Kenya.
A total of 7 master’s degree programs approved by the Commission of University Education are offered at the University of Nairobi and the Moi University School of Medicine: Clinical Pharmacy, Pharmaceutical Analysis, Pharmacoepidemiology and Pharmacovigilance, Industrial Pharmacy, Pharmacognosy and Complementary Medicine, Molecular Pharmacology, and Pharmacology and Toxicology.16
As is the case internationally, for example in Australia,21 the PPB currently recognizes only one level of practice; additional recognition is not given to those who have specialized in multiple pharmacy fields. Pharmacy schools and practitioners have been lobbying for the recognition of holders of postgraduate training as specialists in various fields of pharmacy. At the time of writing, the PPB was working on guidelines for recognition of pharmacy specialists.
Most graduates of the clinical pharmacy program are employed in county hospitals, particularly those in urban and semi-urban areas. In many of these hospitals, other health care providers acknowledge the valuable contribution of clinical pharmacists to the health care team. This has increased demand for practitioners in this field. Currently, none of these pharmacists have prescribing rights, which limits their current role.
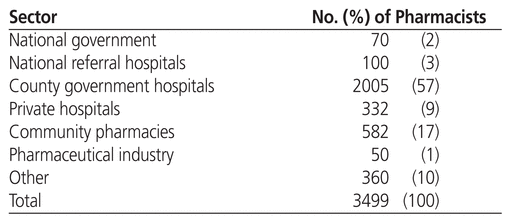
Table 1 shows the numbers of pharmacists in various sectors of pharmacy practice in Kenya. Of the country’s 3499 registered pharmacists (in 2013), about 70% worked in the hospital sector.14 Pharmacists working in hospitals have various duties and responsibilities,22,23 including the following:
administrative positions, such as medical superintendents (hospital chief executive officers), department heads, pharmacy leaders;
management of the medicine-use cycle, specifically quantification, procurement (ordering), distribution, and administration;
secretary to the medicines and therapeutics committee;
dispensing of medicine, extemporaneous preparation, sterile and nonsterile compounding;
provision of prescriber and patient education through continuing medical education programs and counselling or patient talks;
pharmacovigilance activities (e.g., setting up reporting systems for poor-quality medicines, identification and documentation of adverse reactions to medicines, and monitoring for medication errors);
hospital-based research, such as medicine utilization reviews.
Table 1 Distribution of Pharmacists in Kenya (2013)14
The national government employs a small percentage of pharmacists for policy-making, standards and quality assurance, national disease programs, and within the PPB. Pharmacists may also be deployed to other ministries working at the pharmaceutical desks, ports of entry, regional and international health secretariats, and semi-autonomous governmental agencies.22 A certain proportion of pharmacists are found in academia, conducting lectures and research in universities, tertiary colleges, nongovernmental organizations, and research institutions.
About 17% of pharmacists currently practise in community pharmacies. Similar to the situation in hospital pharmacy practice, efforts are under way, albeit slowly, to move from traditional product-based community pharmacy to a more patient-focused model.24 Pharmacists in community practice are taking time to build their clinical skills in response to market needs. Pharmacists in this setting generally act in a managerial capacity or as superintendents. Their role revolves around supervising junior staff, supply-chain management, extemporaneous compounding, and dispensing of medicines.
Pharmacists working in the pharmaceutical industry are mainly employed to handle tasks related to regulatory affairs, quality assurance, production, drug development, and marketing. This group accounts for a very small percentage (just over 1%) of registered pharmacists.
In summary, most pharmacists are employed in county hospitals, which are run by the county governments. The community pharmacy sector has relatively few pharmacists because it is more product-oriented. Currently, there is a drive to boost pharmacists’ interest in community pharmacy practice by introducing medication therapy management services into this setting.
In large hospitals, whether private or public, inpatient dispensing is done from satellite pharmacies throughout the hospital, with outpatient services provided from a separate section. Small hospitals typically have one pharmacy from which all medication is dispensed. Figure 1 shows how medicines are distributed in Kenyan public hospitals.25
|
|
||
|
Figure 1 Pharmaceutical supply chain in Kenyan public hospitals. Medicines from the main drug stores are moved to either the inpatient pharmacy or the outpatient pharmacy. The inpatient pharmacy caters to all patients admitted to the various wards and operating rooms, as well as the injectables administration room for patients in the outpatient department (OPD). The outpatient pharmacy is the dispensing point for patients who have attended clinics of the hospital. DDA = Dangerous Drug Act. Reproduced, with permission, from MDS-3: Managing Access to Medicines and Health Technologies (Figure 45-1, page 45.5). © 2012 Management Sciences for Health, Arlington, Virginia. |
||
The PPB is the regulatory authority mandated by law to regulate the practice of pharmacy and the manufacture of and trade in drugs. It is responsible for overseeing standards of safety, efficacy, and quality of all drugs, chemicals, and medical devices used in the country.
All pharmacists who want to practise in Kenya must be registered and licensed by the PPB. Licences to practise are renewed annually, whether the pharmacist is practising in a hospital, in the community, or in industry. Licence renewal depends on obtaining a mandatory minimum number of continuing professional development points. These professional development points may be earned in a number of ways. For example, pharmacy practitioners have formed professional bodies reflecting their various scopes of practice (Table 2). These bodies often organize continuing professional development sessions to ensure that pharmacists keep abreast of new advances in their field. The Pharmaceutical Society of Kenya organizes online sessions and an annual scientific conference. The Hospital Pharmacists Association of Kenya, which promotes best standards in hospital pharmacy practice, organizes monthly continuing professional development sessions, a quarterly 1-day workshop, and an annual 2-day symposium.
Table 2 Professional Pharmacy Organizations in Kenya
Buildings that will be used for conducting any pharmacy business must be inspected by the PPB to ensure compliance with the relevant requirements. The Pharmaceutical Society of Kenya advocates for the use of a green cross banner outside its members’ outlets, to assist the public in identifying premises that are operated by duly qualified pharmacists.
Manufacturing plants for pharmaceutical drugs have to comply with current good manufacturing practices before they are licensed for drug production.26 A certificate of analysis must be presented, together with the application for registration of medications. Analysis of samples is usually done in accredited laboratories within Kenya and the East African region, such as the National Quality Control Laboratory, the Mission for Essential Drugs and Supplies (MEDS) quality control laboratory, and the University of Nairobi Drug Analysis and Research Unit.27
Access to health care in Kenya is a fundamental human right, as enshrined in the country’s constitution.4 It was with this consideration in mind that the main aim of the Kenya National Pharmaceutical Policy was set as “Universal access to quality essential medicines, essential health technologies and pharmaceutical services in Kenya”.28
The Kenya Essential Medicines List29 provides a guide as to which medications should be stocked, especially in public facilities; some hospitals have also developed their own medicine formularies to suit their specific needs.30 The Ministry of Health procures and distributes medicines that are used for malaria, tuberculosis, sexually transmitted diseases, HIV/AIDS, and family planning programs, because these medications are funded through international partners (at the national level).31 The county governments are responsible for procuring medicines for the facilities under their jurisdiction (other than those procured by the Ministry of Health). Medicines for other conditions, such as antibiotics, are sourced from private wholesale suppliers, nongovernmental organizations (such as MEDS), and KEMSA.32,33 KEMSA and MEDS have a wide range of products available and lower prices than private wholesalers. In the private sector and faith-based health facilities, the availability of medicines and their prices are higher than in the public sector.34
In public health facilities, only 40% of essential medicines are available at any one time, and supply problems are common28,31,34,35 because of inadequate funding and inappropriate selection and irrational use of the available medicines.31,36 These problems occur despite guidance on the appropriate use of medication in public hospitals provided in standard treatment guidelines. Hospitals are also mandated to have Medicines and Therapeutics Committees that are charged with the responsibility of ensuring rational use of medicines in their institutions.37
In public primary care facilities, health care, including medicines, is provided free of charge, with patients paying only minimal registration fees. Children under 5 years of age are entitled to free health care (including medicines) in public and faith-based health care facilities, and a waiver system is in place for patients older than 5 years of age who cannot afford treatment. Publicly procured medicines for priority health programs, such as those for contraception, malaria, HIV/AIDS, and tuberculosis, are provided free of charge through public and faith-based health care facilities. Cost-sharing applies for treatment of other conditions in adults and children over 5 years of age, at higher-level public facilities. The private sector provides health services, including medicines, on a full cost-recovery basis. There is currently no policy in Kenya to guide the pricing of medicines in any sector.28,31,38
All medical products, including vaccines, are subject to market authorization by the PPB, which also has a robust pharmacovigilance system, as mentioned earlier.39,40 The PPB also conducts postmarketing surveillance to test the quality of products, in either of the 2 World Health Organization–accredited laboratories in the country. This surveillance allows the quality of medicines to be tracked in the supply chain. Unfortunately, the storage facilities in public facilities, faith-based health facilities, and private pharmacies are inadequate. They are often small, with poor air circulation and rudimentary stock-monitoring systems, which compromises the quality of the medications stored. Large procurement entities such as MEDS and KEMSA have better storage facilities (in this regard, the MEDS warehousing is superior to that of KEMSA).31
The national government’s Ministry of Health provides all routine vaccines free of charge through the Unit of Vaccine and Immunization Services.41 As of 2014, 75% of children aged 12–23 months were fully vaccinated, a level comparable to immunization coverage worldwide.42 Vaccination coverage is determined by birth order, residence, mother’s education, and family income.43 The Unit of Vaccine and Immunization Services and the PPB periodically assess vaccines for conformity with set quality and potency standards. However, there is no specific legislation or guidelines for monitoring vaccine safety and organizational structure.41,44
The conventional technically oriented perception of pharmacy is changing in response to emerging technologies, automation, and patient need. The professional pharmacy role is becoming more clinical, and pharmacists are increasingly regarded as health care providers. Maintaining and justifying this status is vital to survival of the profession in an environment where technology can deliver many traditional pharmacy roles, such as filling prescriptions, and where electronic information systems can provide drug information. To maintain and further develop this clinical health care role, pharmacy needs to be underpinned by appropriate training, needs-based service delivery, and enabling legislation, as described below.
Training programs should be revised to ensure there is competence not only in delivering a product-based service but also in newer areas of clinical practice. Such training will equip the profession with specialization and knowledge that is more valuable to patients than what is available to them online. Increased competency will promote patients’ confidence in the pharmacist and will ensure survival of the profession as the experts in medicines.
The profession will need to use technology and technicians to deliver traditional product-based services, so that pharmacists can spend their time providing pharmaceutical care to patients, in accordance with their needs, at a time and place of the patients’ choosing. The profession should target its services to improving disease outcomes and reducing drug-related problems.
Enabling legislation needs to be introduced to allow pharmacists to undertake new roles such as pharmaceutical care; medication therapy management and contracts need to be revised so that pharmacists receive recognition and remuneration for their new roles. However, there is also a need for evidence confirming the added value that pharmacists bring to the health care team. Local initiatives showcasing extended pharmacy roles will be core to gaining the support of patients and health care practitioners based on first-hand experience. It will then be easier to put appropriate legislation and contracts into place.
1 The world factbook. Washington (DC): Central Intelligence Agency; 2017 [cited 2017 Oct 26]. Available from: https://www.cia.gov/library/publications/resources/the-world-factbook/geos/ke.html
2 Kenya GDP per capita 1960–2017. New York (NY): Trading Economics; 2017 [cited 2017 Oct 26]. Available from: https://tradingeconomics.com/kenya/gdp-per-capita
3 Kenya: factsheets of health statistics. Geneva (Switzerland): World Health Organization; 2016.
4 Republic of Kenya. The Constitution of Kenya. Laws Kenya. 2010:191.
5 Kenya health policy 2014–2030: towards attaining the highest standard of health. Nairobi (Kenya): Ministry of Health; 2014.
6 Kenya national health accounts 2012/2013. Nairobi (Kenya): Ministry of Health; 2015.
7 Kenya: health information, research, evidence and knowledge. Geneva (Switzerland): World Health Organization, African Health Observatory; 2014 [cited 2017 Mar 4]. Available from: www.aho.afro.who.int/profiles_information/index.php/Kenya:Health_information,_research,_evidence_and_knowledge#Data_management
8 Health information system policy. Nairobi (Kenya): Ministry of Medical Services, Ministry of Public Health and Sanitation; 2011.
9 Kihuba E, Gathara D, Mwinga S, Mulaku M, Kosgei R, Mogoa W, et al. Assessing the ability of health information systems in hospitals to support evidence-informed decisions in Kenya. Glob Health Action. 2014;7(1): Article 24859.

10 Pharmacovigilance electronic reporting system. Nairobi (Kenya): Pharmacy and Poisons Board; 2017 [cited 2017 Mar 28]. Available from: www.pv.pharmacyboardkenya.org/
11 Background [website]. Nairobi (Kenya): Kenya Medical Research Institute; 2017 [cited 2017 Mar 28]. Available from: https://www.kemri.org/index.php/features-intro/background
12 Kenyatta National Hospital research publications. Nairobi (Kenya): Kenyatta National Hospital; 2017 [cited 2017 Nov 21]. Available from: http://knh.or.ke/index.php/research-publications/
13 Moi Teaching and Referral Hospital [homepage]. Moi (Kenya): Moi Teaching and Referral Hospital; 2017 [cited 2017 Mar 29]. Available from: www.mtrh.or.ke/
14 Health sector human resources strategy 2014–2018. Nairobi (Kenya): Ministry of Health; 2014.
15 Gathura G. Traditional healers join formal health care in Kenya. Rocket Science; 2017 [cited 2017 Oct 25]. Available from: http://rocketscience.co.ke/2017/07/17/traditional-healers-join-formal-health-care-in-kenya/
16 Ogaji IJ, Kahiga TM, Gachuno OW, Mwangi JW. Development of pharmacy education in Kenya universities to date. Afr J Pharm Pharmacol. 2016;10(18):385–92.
17 Pharmaceutical Society of Kenya [homepage]. Nairobi (Kenya): Pharmaceutical Society of Kenya; 2017 [cited 2017 Mar 29]. Available from: http://psk.or.ke/
18 Registered institutions to offer pharmacy in Kenya. Nairobi (Kenya): Kenyapharmtech; 2017 [cited 2017 Mar 29]. Available from: http://kenyapharmtech.com/registered-institutions-to-offerpharmacy-course-in-kenya/
19 Kunz AH. Purdue Kenya Partnership honored with prestigious regional award. Purdue Univ News. 2014 Nov 4 [cited 2017 Mar 29]. Available from: https://www.purdue.edu/newsroom/releases/2014/Q4/purduekenya-partnership-honored-with-prestigious-regional-award.html
20 East African Kidney Institute (EAKI). Nairobi (Kenya): University of Nairobi, College of Health Sciences; 2017 [cited 2017 Mar 29]. Available from: http://chs.uonbi.ac.ke/node/9408
21 Moles RJ, Stehlik P. Pharmacy practice in Australia. Can J Hosp Pharm. 2015;68(5):418–26.

22 Proposed scheme of service for pharmacists. Nairobi (Kenya): Ministry of Health; 2012.
23 Ombaka E, Kusemererwa D, Meiburg A. Pharmacy practice in church health institutions: minimum standards for hospitals. Nairobi (Kenya): Ecumenical Pharmaceutical Network; 2011.
24 Smith F. Private local pharmacies in low- and middle-income countries: a review of interventions to enhance their role in public health. Trop Med Int Health. 2009;14(3):362–72.
25 MDS-3: Managing access to medicines and health technologies. Arlington (VA): Management Sciences for Health; 2012.
26 Registration of drugs: guidelines to submission of applications. Nairobi (Kenya): Pharmacy and Poisons Board; 2010.
27 Manyuru P. Improving supply chain for achieving universal health coverage (UHC): experience from MEDS, Kenya [slide presentation]. African Comprehensive HIV/AIDS Partnerships 7th Biennial Conference; 2015 Feb 25 [cited 2017 Nov 21]; Nairobi (Kenya). Available from: https://www.slideshare.net/achapkenya/experience-from-meds-kenya-by-pascal-manyuru
28 Kenya national pharmaceutical policy. Nairobi (Kenya): Ministry of Medical Services, Ministry of Public Health and Sanitation; 2010.
29 Kenya essential medicines list 2016. Nairobi (Kenya): Ministry of Health; 2016.
30 Otieno Y. Kenya’s national hospital develops country’s first-ever drug formulary. Management Sciences for Health; 2013 Oct 30 [cited 2016 Jun 27]. Available from: https://www.msh.org/news-events/stories/kenyas-national-hospital-develops-countrys-first-ever-drug-formulary
31 Access to essential medicines in Kenya: a health facility survey. Nairobi (Kenya): Ministry of Public Health and Sanitation, Ministry of Medical Services; 2009.
32 MEDS County Government Partnerships Initiative 2013. Nairobi (Kenya): Mission for Essential Drugs and Supplies; 2013 [cited 2016 Jun 27]. Available from: www.meds.or.ke/index.php/about-us/our-partnerships
33 KEMSA new business model. Nairobi (Kenya): Kenya Medical Supplies Authority; 2013 [cited 2016 Jun 27]. Available from: www.kemsa.co.ke/index.php?option=com_content&view=article&id=72&Itemid=148
34 Kenya pharmaceutical country profile. Nairobi (Kenya): Ministry of Medical Services, World Health Organization; 2010.
35 Mecca LW, Riungu J, Guantai EM. Financing and availability of essential medicines before and after introduction of the National Hospital Insurance Fund Civil Servants and Disciplined Services Medical Scheme at Webuye District Hospital, Kenya. Afr J Pharmacol Ther. 2014;3(4):128–33.
36 Wangu MM, Osuga BO. Availability of essential medicines in public hospitals: a study of selected public hospitals in Nakuru County, Kenya. Afr J Pharm Pharmacol. 2014;8(17):438–42.
37 Clinical management and referral guidelines. Volume III: Clinical guidelines for management and referral of common conditions at levels 4–6: hospitals. Nairobi (Kenya): Ministry of Medical Services, Ministry of Public Health and Sanitation, World Health Organization; 2009.
38 Medicines prices in Kenya. Geneva (Switzerland): World Health Organization, Health Action International Africa; 2004.
39 Guidelines for the national pharmacovigilance system in Kenya. Nairobi (Kenya): Ministry of Medical Services, Ministry of Public Health and Sanitation, Pharmacy and Poisons Board; 2009.
40 Otieno Y. Pharmacovigilance reporting goes digital in Kenya. Management Sciences for Health; 2013 Apr 29 [cited 2016 Jul 4]. Available from: https://www.msh.org/news-events/stories/pharmacovigilance-reporting-goes-digital-in-kenya
41 National policy guidelines on immunization 2013. Nairobi (Kenya): Ministry of Health; 2013.
42 Immunization coverage: fact sheet. Geneva (Switzerland): World Health Organization; 2017 Jul [cited 2017 Oct 25]. Available from: www.who.int/mediacentre/factsheets/fs378/en/
43 Kenya demographic and health survey 2014. Nairobi (Kenya): Kenya National Bureau of Statistics, Ministry of Health, National AIDS Control Council, Kenya Medical Research Institute, National Council for Population and Development, DHS Program ICF International; 2015 Dec.
44 Kugo CL. The vaccine pharmacovigilance of Kenya [thesis]. Nairobi (Kenya): University of Nairobi; 2016.
Competing interests: None declared. ( Return to Text )
The authors would like to thank Senior Pharmacist Fellow Dr Harshvadan Maroo, FPSK, FRPharmS, and the national officials of the Hospital Pharmacists Association of Kenya (HOPAK) for their advice and unwavering support in the writing of this article.
Canadian Journal of Hospital Pharmacy, VOLUME 70, NUMBER 6, November-December 2017