Pierre-André Dubé, MSc
Antidotes play an essential role in the treatment of many poisonings and must be readily available for timely administration. Although there is expert consensus concerning guidelines on stocking of antidotes (e.g., Dart and others1), studies have consistently shown insufficient inventory of antidotes in Canadian emergency care hospitals.2–5 This letter describes some of the initiatives that have emerged across Canada to ensure adequate stocking of antidotes, which have ultimately led to development of the Canadian Antidote Registry.
For many decades, the British Columbia Drug and Poison Information Centre (DPIC) operated a clinical antidote program for digoxin immune Fab (starting in 1986) and fomepizole (starting in 2002).6,7 Upon consultation, the DPIC would provide free antidote replacement to the treating hospital. This approach ensured that patients received the antidotes after consultation with a toxicology expert, which minimized costs and allowed DPIC to collect province-wide usage data. The program was discontinued in April 2017. Now, BC hospitals must purchase their own stock of antidotes, which makes it easier for each hospital to control its inventory.
Between 2002 and 2004, the West Parry Sound Health Centre began to operate the Massasauga Rattlesnake Antivenom Depot (https://www.wpshc.com/index.php/ontario-antivenom-depot-117), which now manages antivenom supplies for depot sites across Ontario (about 150–200 vials total). An advisory group meets twice a year, and protocols are reviewed by the depot’s medical director. The antivenom depot rotates stock across provincial depot sites to avoid expiration of unused vials and to reimburse hospitals that ask for clinical consultations. In the Toronto region, hospital pharmacy directors have, since 2004, signed an annual agreement for the Toronto and Area Hospital Pharmacy Departments Antidote Sharing Strategy. This strategy includes procedures and policies for sharing and borrowing antidotes. Following the death by poisoning of a 25-year-old man,8 the Patient Safety Review Committee of the Office of the Chief Coroner recommended in 2013 that “[h]ospitals should review the antidotes they stock on a regular basis, and at least annually. If a given antidote is not stocked by a hospital, a plan should be in place and readily available to staff in order to ensure that this antidote can be obtained rapidly from another institution or source, on a 24/7 basis.”9
In May 2005, the IWK Regional Poison Centre introduced the Nova Scotia Provincial Antidote Kit Program, through which hospital administrators manage antidote purchases. All tertiary care centres and regional hospital emergency departments stock full kits, whereas community hospitals may stock full or modified kits. Because the province has a small number of hospitals (n = 37), this initiative appears to be viable and provides usage data. In 2016, overall hospital compliance was 89%.10
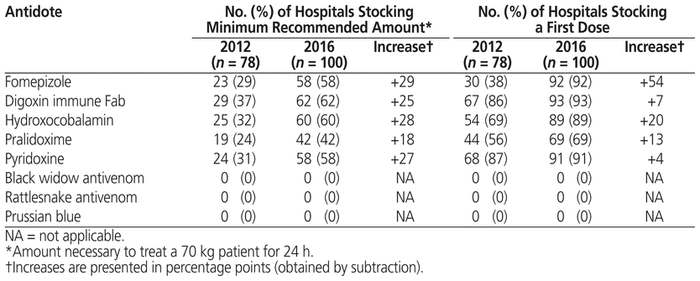
In 2012, the Institut national de santé publique du Québec (the Quebec National Institute of Public Health) created the Quebec Antidote Registry to assist health care professionals.5 This registry is now hosted by the Portail Toxicologie Clinique (Clinical Toxicology Portal; https://www.inspq.qc.ca/toxicologie-clinique/registre-provincial-des-antidotes). It includes all available formulations of antidotes, as selected by a group of experts, and hospital participation is voluntary. An analysis of data from this registry was undertaken recently as part of the background to the Canadian Antidote Registry. The analysis showed that from 2012 to 2016, an average of 85% (93/109) of hospitals providing emergency care services in the province participated in the registry. Over the same period, for many antidotes the percentage of hospitals stocking the first dose or the minimum recommended amount increased (Table 1), although a few antidotes remain unavailable. This small study is the first to report a substantial increase in hospitals’ adherence to the recommendations of the Centre antipoison du Québec (Quebec Poison Centre) for antidote stockpiling in the province.
Table 1 Stocking of Minimum Recommended Amount of First Dose of Selected Antidotes by Quebec Hospitals Providing Emergency Services (2016 versus 2012)
In March 2012, the New Brunswick Drugs and Therapeutics Committee adopted its Antidote Policy.11 All 19 NB hospitals with an emergency department must stock and maintain a quota of antidotes, with 8 of these hospitals being defined as depot sites. The provincial Antidote Committee reviews the antidote list and associated drug monographs every 12–18 months.
In 2014, Alberta Health Services Pharmacy Services developed the Alberta Antidote Stocking Recommendations, which are reviewed every 1–2 years. Unlike typical poison centre recommendations, these have been approved by the Provincial Drugs and Therapeutics Committee, which makes them mandatory for all hospitals in the province. However, no data are available on hospitals’ compliance with the recommendations.
According to the successful model already in place in Quebec, a preliminary version of the Canadian Antidote Registry was developed, following recommendations made by the experts involved in the programs and initiatives described above. The application will be hosted on the Canadian Network for Public Health Intelligence platform (https://www.cnphi-rcrsp.ca/cnphi/index.jsp) and will include a Web-based formulary to more effectively collect the data. Eventually, hospitals will be asked to report their inventories of antidotes to the national registry, at least twice a year and following any changes in their quotas. In the coming months, one Provincial Antidote Registry (PAR) Coordinator will be appointed by each province or territory. Also, within each jurisdiction, a Local Antidote Registry (LAR) Coordinator will be appointed for each hospital or health establishment that stocks antidotes. The experts working on the national registry have agreed that all PAR and LAR Coordinators should be hospital pharmacists.
A pilot project will be conducted by March 31, 2018, in selected hospitals. Depending on the adjustments that need to be carried out, the Canadian Antidote Registry should be operational by fall 2018. Meanwhile, the “Canadian Antidote Guide in Acute Care Toxicology” should be available online and on mobile apps by summer 2018. This guide will complement the registry and will help health care professionals in managing proper antidote administration to their patients. It can be concluded that the expertise of hospital pharmacists will be required in the coming months and years to improve the quality of care provided to patients who experience poisoning.
1 Dart RC, Goldfrank LR, Erstad BL, Huang DT, Todd KH, Weitz J, et al. Expert consensus guidelines for stocking of antidotes in hospitals that provide emergency care. Ann Emerg Med. 2017. Available from: http://dx.doi.org/10.1016/j.annemergmed.2017.05.021 [Epub ahead of print].
2 Bailey B, Bussières JF. Antidote availability in Quebec hospital pharmacies: impact of N-acetylcysteine and naloxone consumption. Can J Clin Pharmacol. 2000;7(4):198–204.
3 Wiens MO, Zed PJ, Lepik KJ, Abu-Laban RB, Brubacher JR, Gorman SK, et al. Adequacy of antidote stocking in British Columbia hospitals: the 2005 Antidote Stocking Study. CJEM. 2006;8(6):409–16.
4 Gorman SK, Zed PJ, Purssell RA, Brubacher J, Willis GA. Antidote stocking in British Columbia hospitals. CJEM. 2003;5(1):12–7.
5 Dubé PA, Lord MC, Bussières JF, Courteau M. Conformité des établissements de santé du Québec aux recommandations de stockage des antidotes. Pharmactuel. 2013;46(3):221–7. Article in French, with English abstract.
6 Gair R, Kent DA, Purssell R, Copland M. Chronic digoxin toxicity in elderly British Columbians. BC Med J. 2011;53(9):490–1.
7 Gair R. Antidote program in British Columbia [letter]. Am J Health Syst Pharm. 2012;69(13):1108.
8 File no. 2012-7219 (PSRC-201 3-01) [case report of Patient Safety Review Committee]. Toronto (ON): Office of the Chief Coroner for Ontario, Patient Safety Review Committee; 2013 [cited 2018 Jan 16]. Available from: http://www.justiceforjosh.com/patientsafetya.PDF
9 Patient Safety Review Committee 2013–2014 annual report. Toronto (ON): Office of the Chief Coroner Province of Ontario; 2015 Nov [cited 2016 Oct 12]. Available from: https://www.oha.com/Documents/2013-14%20PSRC%20Annual%20Report%20-%20English.pdf
10 Murphy NG, Bona DR, Hurley TA. A system-wide solution to antidote stocking in emergency departments: the Nova Scotia antidote program. CJEM. 2017. doi: 10.1017/cem.2017.400. [Epub ahead of print].

11 Provincial Drugs and Therapeutics Committee. Antidotes policy. New Brunswick Regional Health Authorities; 2015 Jun.
Competing interests: None declared. ( Return to Text )
Funding: The Canadian Antidote Registry has received financial, technical, and professional support from the Government of Canada, through Defence Research and Development Canada, the Public Health Agency of Canada, and Health Canada. The Government of Canada was not involved in the design and conduct of this report; the collection, management, or interpretation of any data; or the preparation, review, or approval of the manuscript. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 71, NUMBER 1, January-February 2018