Adriel Chan, Libby Liang, Anthony C H Tung, Angus Kinkade, Aaron M TejaniABSTRACT
Background
The use of proton pump inhibitors (PPIs) may cause significant harm to patients in the residential care setting, as these patients are often frail with multiple morbidities. The extent of non–evidence-based use of PPIs in residential care sites of the Fraser Health Authority in British Columbia is unknown.
Objective
To determine the proportion of non–evidence-based use of PPI therapy for residential care patients of the Fraser Health Authority.
Methods
This retrospective cross-sectional study was conducted in 6 Fraser Health residential care facilities in British Columbia between April 1, 2015, and March 31, 2016. Two definitions of “evidence-based indications” were used. The first definition encompassed broad evidence-based indications for PPI use, specifically gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), gastritis, esophagitis, Barrett esophagus, and gastrointestinal protection from concurrent oral steroids, oral nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. The second definition involved common evidence-based indications for PPI use, specifically GERD or PUD. Descriptive statistics were used to evaluate the primary outcome: the proportion of PPI orders without a documented broad or common evidence-based indication for PPI treatment.
Results
A total of 331 residential care patients and 407 PPI orders were assessed. The proportion of PPI orders without a documented broad evidence-based indication was 16.2% (66/407). The proportion of PPI orders without a documented common evidence-based indication was 43.7% (178/407). The most frequently documented reason for a PPI order was GERD (214/407 or 52.6%). PPI orders for patients with GERD and gastrointestinal bleeding had the longest duration of therapy during residential care admission, averaging 205.1 and 218.1 days, respectively.
Conclusion
About 1 in 6 PPI orders for Fraser Health residential care patients did not have a documented broad evidence-based indication, and about 2 in 5 PPI orders did not have a documented common evidence-based indication. These results indicate a need to assess the appropriateness of therapy for every patient with an active PPI order in residential care facilities.
KEYWORDS: evidence-based care, proton pump inhibitor, residential care
RÉSUMÉ
Contexte
L’emploi d’inhibiteurs de la pompe à protons (IPP) peut causer des torts importants aux patients qui résident en centre d’hébergement et de soins de longue durée, car souvent ces personnes sont fragiles et souffrent de multiples maladies. On ignore quelle est la proportion d’utilisation d’IPP ne reposant pas sur des données probantes dans les centres d’hébergement et de soins de longue durée de la Fraser Health Authority en Colombie-Britannique.
Objectif
Déterminer la proportion d’utilisation de traitement par IPP ne reposant pas sur des données probantes chez les patients en centre d’hébergement et de soins de longue durée de la Fraser Health Authority.
Méthodes
Cette étude rétrospective transversale a été menée dans six centres d’hébergement et de soins de longue durée de la Fraser Health en Colombie-Britannique, entre le 1er avril 2015 et le 31 mars 2016. Deux définitions du terme « indications fondées sur des données probantes » ont été utilisées. La première définition englobait des indications larges fondées sur des données probantes appuyant l’utilisation d’IPP, plus particulièrement : pour traiter le reflux gastro-œsophagien, l’ulcère gastroduodénal, la gastrite, l’œsophagite et l’œsophage de Barrett ainsi que pour fournir une protection gastrique contre les effets indésirables de la prise de médicaments anti-inflammatoires oraux stéroïdiens ou non stéroïdiens, d’antiplaquettaires et d’anticoagulants. La seconde définition comprenait les indications usuelles fondées sur des données probantes pour appuyer l’utilisation d’IPP, plus précisément : le reflux gastro-œsophagien ou l’ulcère gastroduodénal. Des statistiques descriptives ont été employées pour analyser le principal paramètre d’évaluation : la proportion d’ordonnances d’IPP pour lesquelles aucune indication, large ou usuelle, fondée sur des données probantes n’a été consignée.
Résultats
Au total, les dossiers de 331 résidents de centres d’hébergement et de soins de longue durée et 407 ordonnances d’IPP ont été évalués. La proportion d’ordonnances d’IPP pour lesquelles aucune indication large fondée sur des données probantes n’a été consignée était de 16,2 % (66/407). La proportion d’ordonnances d’IPP pour lesquelles aucune indication usuelle fondée sur des données probantes n’a été consignée était de 43,7 % (178/407). La raison la plus souvent consignée pour l’émission d’une ordonnance d’IPP était le reflux gastro-œsophagien (214/407 ou 52,6 %). Les ordonnances d’IPP destinées aux patients souffrant de reflux gastro-œsophagien ou d’hémorragie gastro-intestinale étaient celles pour lesquelles la durée du traitement était la plus longue au cours du séjour en centre d’hébergement et de soins de longue durée, soit respectivement de 205,1 et 218,1 jours en moyenne.
Conclusion
Environ 1 ordonnance d’IPP sur 6 pour les patients de centres d’hébergement et de soins de longue durée de la Fraser Health ne reposait pas sur une indication large consignée et fondée sur des données probantes et environ 2 ordonnances d’IPP sur 5 ne s’appuyaient pas sur une indication usuelle consignée et fondée sur des données probantes. Les résultats révèlent la nécessité d’évaluer la pertinence des traitements par IPP pour chaque patient ayant une ordonnance active d’IPP dans les centres d’hébergement et de soins de longue durée.
MOTS CLÉS: soins basés sur les données probantes, inhibiteur de la pompe à protons, centre d’hébergement et de soins de longue durée
Proton pump inhibitors (PPIs) are a class of drugs used to treat various gastrointestinal (GI) conditions, such as gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), Helicobacter pylori infections, Barrett esophagus, esophagitis, and gastritis.1,2 PPIs work by selectively and irreversibly inhibiting hydrogen potassium ATPase on parietal cells, thereby leading to decreased gastric acid levels in the stomach.3,4 PPIs are considered relatively safe, their most frequent adverse effects being headache and GI-related problems (nausea, abdominal pain, flatulence, constipation, and diarrhea).4 Long-term use of PPIs is associated with serious complications such as Clostridium difficile infections, pneumonia, vitamin B12 deficiency, and calcium deficiency, the last of which increases the risk of osteoporosis and associated bone fractures.4–6
In a US study that assessed 355 600 nursing home residents aged 65 or older (representing 27% of the total population of such residents) who were receiving at least 1 PPI, 49% of those evaluated did not have a documented evidence-based indication for the drug.1 No similar studies have been done within the residential care facilities of the Fraser Health Authority in British Columbia, such that the proportion of these patients without an evidence-based indication for PPI treatment is largely unknown. Residential care patients have more comorbidities than the general public and are therefore at increased risk of polypharmacy.7 Inappropriate prescribing of PPIs is an issue associated with polypharmacy and puts residential care patients at increased risk of PPI-induced complications. Therefore, it is important to investigate the local proportion of inappropriate PPI use, to support PPI deprescribing efforts and potentially reduce the risk of complications.6,8
The primary objective of this study was to determine the proportion of PPI orders among Fraser Health residential care patients for which a broad evidence-based indication or a common evidence-based indication could not be identified. The secondary objectives were to determine the proportion of PPI orders with no documented indication of any kind, the proportion of PPI orders for patients with a history of PPI use before admission to residential care, the documented indications for PPI therapy, and the duration of PPI therapy.
This retrospective, cross-sectional study examined non—evidence-based use of PPIs at Fraser Health residential care sites between April 1, 2015, and March 31, 2016. The Fraser Health Authority is a large, publicly funded health authority in British Columbia that provides a variety of health care services in the Fraser region of the province, from Burnaby to Boston Bar. As part of this mandate, Fraser Health serves a total of 7760 residents at the following 6 publicly owned and funded residential care facilities (all of which maintain electronic medical records): Chilliwack General Hospital, Delta Hospital, Langley Memorial Hospital, Mission Memorial Hospital, Peace Arch Hospital, and Queen’s Park Care Centre. Data from these facilities were collected for this study in June 2016.
All of the publicly funded residential care facilities included in this study had an assigned clinical pharmacist. The clinical pharmacist is required to conduct a medication review every 6 months for every patient and also is expected to deal with medication-related problems on a daily basis. The average length of stay for patients in all facilities is 2.6 years, and the average frailty of patients at the time of this study, as measured by the Clinical Frailty Index,9 was 7 (unpublished data). Medication reconciliation on admission is conducted at some sites but is not a requirement for all sites.
One investigator (L.L) identified all PPI orders during the study period and assigned them to 2 investigators (A.C., L.L.) for screening, application of inclusion and exclusion criteria, and data collection. Patients were identified through the pharmacy’s order-entry system, Meditech, from a report of all PPI orders during the study period. Patients from the 6 Fraser Health–owned and operated residential sites were included if they had at least 1 PPI order during the study period. For patients with multiple courses of PPI treatment, a pair of PPI prescriptions was considered to represent separate courses of therapy if more than 7 days had elapsed between the 2 orders; conversely, a PPI order started within 7 days of discontinuation of another PPI order was considered to be a continuation of the earlier order. Patients for whom no electronic charts were available were excluded.
Some patients had received “pass medications”, a supply of prescribed PPIs to take with them when they were away from their respective residential care sites (e.g., during a visit home). A pass PPI order was defined as a PPI order with a duration of 7 days or less that was prescribed concurrent with ongoing PPI therapy. These orders, identified in the patient charts, were excluded from the study because they were considered to be duplicate orders for patients who were receiving active PPI treatment while in residential care.
The investigators collaborated to create an electronic data extraction form. The following data were collected from medical records and recorded using this electronic form: sex, residential care site, age at the start of PPI treatment during the study period, history of PPI use, start and end dates of PPI treatment, regimen (drug, dose, frequency, total daily dose) of the most recent course of PPI therapy, relevant concurrent medications (oral steroids, oral nonsteroidal anti-inflammatory drugs, antiplatelet agents, anticoagulants, bisphosphonates, histamine 2 receptor antagonists), documented indications for PPI therapy, and the date of the most recent upper or unspecified GI bleed, if applicable.
Data from the patient’s entire electronic medical record (accessed through the local health records software) were extracted independently by the 2 investigators (A.C., L.L.) for an initial, randomly selected sample of 50 patient charts. Specific sections of the medical record that were reviewed included the prescriber progress notes, nursing notes, medication administration record, and physician orders. The 2 investigators compared their results to confirm consistency and accuracy of data collection. On the basis of this preliminary comparison, all investigators decided that A.C. and L.L. could each extract data for 50% of the remaining patients without independent duplication. Consultation with the other researchers (A.C.H.T., A.K., A.M.T.) was to occur if A.C. and L.L. encountered problems during data extraction.
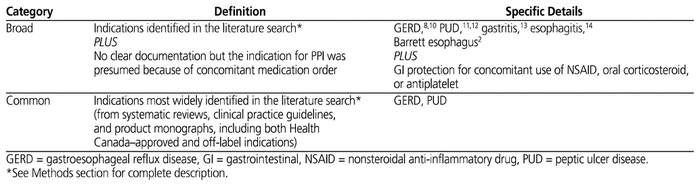
For the purposes of the study, 2 definitions were developed, one for “broad evidence-based indications” for PPIs and the other for “common evidence-based indications” for PPIs (Table 1). To ensure that all possible indications were captured, 2 of the investigators (L.L, A.C.) included Health Canada–approved indications, as well as off-label, evidence-based indications mentioned in clinical practice guidelines and tertiary references. To give an optimistic estimate of usage within evidence-based indications, we did not consider duration of therapy in the primary assessment (i.e., a PPI order used beyond the approved duration for a particular indication included in one or both of the definitions was counted as having an evidence-based indication). The evidence used to support these indications was not scrutinized, and it was assumed that prescribing clinicians would use similar sources of information to guide their prescribing. This conservative approach would likely underestimate the proportion of PPI orders for which no evidence-based indication was documented. Furthermore, our categorization of indications was not validated nor was it used in other studies. The categorization was intentionally broad, to mimic the practical way in which clinicians might think about the indications for use of PPIs.
Table 1 Definitions of Evidence-Based Indications for Proton Pump Inhibitor Therapy
In instances where the documented indication met the criteria for both the “common” and “broad” evidence-based indications, the PPI order was counted in both categories. The reason for allowing this overlap was to assess both the number of PPI orders that met the strict criteria (i.e., common indications) and the number that met the less rigorous/more pragmatic criteria (i.e., broad indications). With regard to the dual aspects of the primary objective, the number of PPI orders without a clearly documented broad or common evidence-based indication was used as the numerator. For patients with multiple PPI orders, all orders were assessed individually. The proportions of PPI orders without broad or common evidence-based indications were then calculated by dividing the aforementioned numerators by the total number of PPI orders.
The proportion of PPI orders for patients with a history of PPI use was determined by dividing the number of orders for these patients by the total number of PPI orders. A patient was deemed to have a history of PPI use if there was a record of PPI treatment during a previous hospital stay (either residential or acute care) or documentation of a history of PPI use anywhere in the electronic medical record. The average duration of PPI therapy during the residential care admission was calculated for all indications and for each indication separately using start and end dates. The average duration of PPI therapy for each indication was calculated by dividing the total duration of PPI therapy for the specified condition (e.g., sum of durations for all PPI orders for all patients with PUD; the numerator) by the total number of PPI orders for that indication (e.g., total number of PPI orders for all patients with PUD; the denominator). For patients who were taking PPIs before admission to any of the residential care facilities, we recorded only that prior PPI therapy had occurred; we did not assess the duration of such prior PPI therapy. Although the duration of PPI therapy before admission to residential care would have added important context, we did not have ready access to data for prescription drug use in the community.
Descriptive statistics were used. Specifically, 95% confidence intervals (CIs) were calculated for all categorical variables (proportion of PPI orders for residential care patients at Fraser Health residential care sites with no evidence-based indication, proportion of PPI orders for patients who were taking PPIs before admission to residential care, and documented reasons for PPI therapy in the identified patients) to estimate the possible range of these outcomes across all Fraser Health residential care sites. The standard deviation (SD) was calculated for the continuous variable (duration of PPI treatment for each patient during the period of residential care).
A total of 674 PPI orders were initially identified by searching the Meditech system. After removal of pass medication orders, duplicate orders, and orders that were considered to be continuous with an earlier order, 410 PPI orders (for 334 patients) remained and were eligible for inclusion in the study. After the removal of orders for 3 patients who had no electronic charts available, 407 orders were considered in the final analysis.
The mean age of the patients in the study was 82 (SD 10) years. Of the initial 410 PPI orders, 154 (37.6%) were for men and 256 (62.4%) were for women. The reason for this difference between the sexes in number of PPI orders was not investigated, nor could it be easily explained by facility type; none of the residential care sites specifically caters to a particular population (e.g., a veterans’ facility).
About 1 of every 6 PPI orders for Fraser Health residential care patients (16.2%) did not have a documented broad evidence-based indication for PPI therapy, and about 2 out of every 5 orders (43.7%) did not have a documented common evidence-based indication (Table 2).
Table 2 Proportion of PPI Orders at Fraser Health Residential Care Sites without Evidence-Based Indications of Various Types (n = 407 Orders)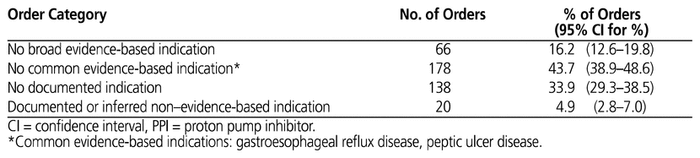
A total of 138 (33.9%) orders had no documented indication at all. Overall, 357 orders (87.7%; 95% CI 83.8%–90.3%) involved patients who had a history of PPI use before their residential care admission. GERD was the most frequently documented reason for PPI therapy, with more than 50% of orders for patients with a documented history of this disease, followed by GI bleeding and PUD (Table 3). A smaller proportion of orders were for patients with a documented history of gastritis, esophagitis, or Barrett esophagus. PPI orders for patients with GERD and GI bleed had the longest duration of therapy, averaging 205.1 and 218.1 days, respectively (Table 4).
Table 3 Documented Reasons for PPI Therapy (n = 407 Orders)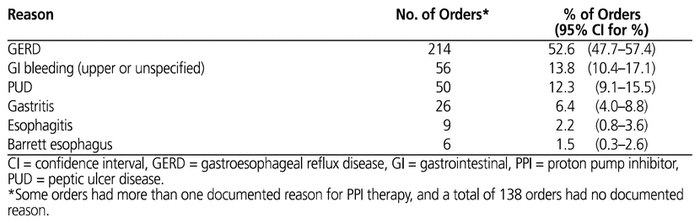
Table 4 Duration of PPI Treatment for Orders during Residential Care Stay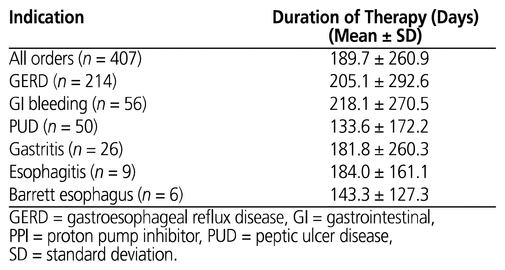
In this study, 16.2% of PPI orders for residential care patients did not have a documented broad evidence-based indication, and 43.7% did not have a documented common evidence-based indication. In addition, 33.9% of PPI orders did not have any documented indication at all. The average duration of PPI orders was about 190 days. These findings highlight some important problems and potential opportunities to improve the use of PPI, as discussed below.
According to our definition of broad evidence-based indications, the findings of this study showed a lower proportion of non–evidence-based PPI use for residential care patients than was reported by Rane and others.1 However, the current findings presumably reflect what would be found at other, privately owned residential care sites within the Fraser Health region, given that these sites implement similar practices and protocols with regard to patient care and medication review.
There could be several reasons for the difference in results between our study and that of Rane and others.1 First, since 2004, the year in which Rane and others1 conducted their analysis, there has been increasing research and awareness about the serious risks associated with PPI use, which may have led to more judicious use of PPIs in the Fraser Health facilities. Another reason could be the existence of regular medication review at Fraser Health residential care sites. It is mandatory that all residential care facilities in British Columbia have a medication safety and advisory committee, consisting of a pharmacist and other health care professionals involved in direct patient care. These committees conduct interdisciplinary meetings to assess medical conditions and drug therapy for all patients (every 6 months for each patient, on a staggered schedule).15
GERD was the most frequently documented reason for PPI therapy, with more than 50% of patients having a documented history of this disease, followed by 13.8% with GI bleeding and 12.3% with PUD. Given that GERD and GI bleeding were the 2 most frequently documented indications for PPI use and given that patients with these conditions had the longest average duration of PPI therapy (205.1 and 218.1 days, respectively), health care providers may be able make the biggest initial impact on reducing inappropriate PPI use by monitoring and assessing the duration of therapy for patients with a history of either of these 2 conditions. The long durations of PPI treatment for GERD and GI bleeding in this study suggest that some patients’ PPI therapy was continued much longer than recommended by guidelines. For example, for the management of symptomatic GERD, the recommended duration of PPI treatment is 4 to 8 weeks; if the patient has an adequate response, the regimen can be changed to an as-needed basis or tapered until discontinuation.6,12 Patients with a long duration of PPI therapy (e.g., more than 8 weeks) should be reassessed and monitored, with a view to tapering the medication.
Notably, the proportion of orders for patients with a history of PPI use before residential care admission was high (357 orders, 87.7%). This number may be an underestimate because PharmaNet records were inaccessible for the purposes of this study. PharmaNet is a data network that links all pharmacies in British Columbia, allowing pharmacists to access patients’ comprehensive medication history. The large proportion of orders involving patients with a history of PPI therapy may suggest that prescribers were simply continuing courses of PPI therapy based on patients’ previous medication histories, without proper assessment of therapy appropriateness. As a result, it is likely that the duration of therapy for a certain proportion of the PPI orders was longer than recommended by guidelines, and the large standard deviation for duration of PPI orders is likely due to several orders with durations of about 1 year or longer. However, given the retrospective nature of this study, we could not assess the appropriateness of therapy for PPIs initially prescribed before admission to residential care. In light of this high proportion of prior PPI utilization, we suggest that health care providers assess each patient’s PPI therapy thoroughly at the time of residential care admission and consider discontinuation, dose tapering, or careful monitoring of the drug. Our findings show that a significant portion of patients received long-term PPI therapy because of a distant diagnosis of conditions such as GI bleeding. In such cases, continuing the use of PPIs may not be the best clinical or therapeutic decision. From a practical perspective, our recommendation could be applied in the clinical setting with little difficulty, as several guidelines exist to provide useful advice for monitoring and deprescribing of PPIs.6,8,16
One finding of particular interest was that 33.9% of PPI orders did not have any documented indication. The absence of a documented indication raises the question of how the clinical team will assess the effectiveness, safety, or appropriateness of therapy. It is imperative that all medications have a clearly documented indication (evidence-based or not) if appropriate monitoring is to take place. This problem can be easily solved with indication-based prescribing.17 According to this approach, when the order for a PPI is written, the indication should be included in the directions for use (e.g., “Take 1 tablet once daily for reflux”). If the indication is mentioned in the directions, the pharmacy will then include this information on the label, and it will also appear in the medication administration record, which becomes part of the patient’s medical record. Additionally, if, after investigation by the clinical team, no indication can be found for an active PPI order, this may be the perfect target for deprescribing in an effort to reduce polypharmacy, especially for those without a documented indication and no symptoms.18
The results of this study can be discussed with policy-makers of the Fraser Health Authority to explore ways to improve the quality of patient care, such as development of a screening tool to be used by physicians and clinical pharmacists to ensure appropriate PPI prescribing. This study investigated only the presence or absence of documented evidence-based indications to determine the appropriateness of PPI therapy, but future studies investigating the regimen and duration specific to the medical condition being treated could help to further optimize PPI prescribing in residential care facilities. It would also be important to evaluate the reasons why indications for PPIs are not being documented in patients’ charts. As mentioned previously, a policy that makes indication-based prescribing the standard would go a long way toward solving the documentation problem.
The definitions for what constitutes an evidence-based indication (either broad or common) were intentionally chosen. This approach would likely underestimate the proportion of PPI orders with truly evidence-based indications.
Several patients had limited or no documentation in their electronic medical records. Poor documentation in patient charts made it difficult to identify the condition the PPI was meant to treat. Inconsistent documentation was also a potential source of bias in this study. For example, a nurse might have documented a patient as having a history of GERD, whereas for the same patient a physician might have documented a history of GI bleeding. Such discrepancies could mask the true indication for PPI treatment. Misinterpretation of clinical events could lead to another potential source of bias. For example, a patient with dyspepsia might have received a diagnosis of GERD. This would be a significant error, as the evidence for PPI treatment in dyspepsia is limited and weaker than the evidence for PPI treatment of GERD.8,19 Another example might be a patient with upper or unspecified GI bleeding for whom the date on which the bleeding stopped was not documented. In this situation, it would not be possible to determine the appropriateness of the duration of PPI therapy.
We identified numerous PPI orders for which no documented reason/indication could be found in the patient’s chart. It would be unfair to conclude that this absence of a documented indication is definitive evidence of inappropriate PPI use. Rather, it would be more appropriate to highlight the lack of clear documentation in the residential care setting. We did not plan to investigate the reasons for poor documentation practices, but this is an important issue that requires further study. Possible questions arising from such an investigation could be, “Why are reasons for medications not documented by health care workers?” and “What is the actual reason that patients with no documented indication for PPI therapy are taking drugs from this class?” These questions were beyond the scope of the current study.
Access to outpatient records was not possible with the resources available. Thus, patients’ complete history of PPI use was unknown. For some patients, completed medication reconciliation forms were included in the electronic medical records, which gave a comprehensive medical history, whereas others had incomplete forms or no forms at all. Also, many patients were taking their own medications, which were not specified in medication lists in their electronic medical records. These medications may have included PPIs or relevant concurrent medications such as nonsteroidal anti-inflammatory drugs or steroids.
Pilot testing with a sample of orders was conducted before data collection for the study began. Two investigators collected data from the same 50 orders and discussed their findings thoroughly to ensure consistency and accuracy of data collection. A more comprehensive method of data collection would have been to have both investigators collect data from all patients independently and compare data to ensure consistency and accuracy. However, because of time limitations, this approach was not possible. Periodic meetings were held with the other researchers involved in the study to resolve any issues regarding data collection.
We calculated the proportion of PPI orders without documented indications, not the proportion of patients who had a PPI order without a documented indication. This approach was useful for patients who had multiple PPI orders for different indications. However, it would lead to overestimation of the proportion of orders without a documented indication if there were multiple orders for the same indication.
About 1 in every 6 patients who was receiving a PPI at Fraser Health residential care sites did not have a documented broad evidence-based indication for the drug (16.2%), and about 2 in every 5 did not have a documented common evidence-based indication (43.7%). Equally concerning was that 33.9% of orders for PPIs did not have any documented indication at all. These findings indicate the need to assess the appropriateness of PPI therapy for every patient with an active PPI order in residential care facilities. Although the results of this study cannot be generalized beyond Fraser Health residential care facilities, the methods used and the findings obtained may be useful for similar assessments in other jurisdictions.
1 Rane PP, Guha S, Chatterjee S, Aparasu RR. Prevalence and predictors of non-evidence based proton pump inhibitor use among elderly nursing home residents in the US. Res Social Adm Pharm. 2017;13(2):358–63.
2 Falk GW. Updated guidelines for diagnosing and managing Barrett esophagus. Gastroenterol Hepatol (NY). 2016;12(7):449–51.
3 Bavishi C, DuPont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34(11–12):1269–81.
4 Ali T, Roberts D, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med. 2009;122(10):896–903.
5 Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med. 2016;176(2):172–4.
6 Farrell B, Pottie K, Thompson W, Boghossian T, Pizzola L, Rashid FJ, et al. Deprescribing proton pump inhibitors. Evidence-based clinical guideline. Can Fam Physician. 2017;63(5):354–64.

7 Harriman K, Howard L, McCracken R. Deprescribing medication for frail elderly patients in nursing homes: a survey of Vancouver family physicians. B C Med J. 2014 Nov [cited 2016 Aug 21];56(9):436–41. Available from: https://www.bcmj.org/articles/deprescribing-medication-frail-elderly-parients-nursing-homes-survey-vancouver-family
8 PPI deprescribing: approaches for stopping or dose reduction of PPIs in those who may not need lifelong treatment. In: RxFiles. Saskatoon (SK): Saskatchewan Health Authority; 2015 Apr [cited 2016 Aug 21]. Available from: http://www.rxfiles.ca/rxfiles/uploads/documents/PPI-Deprescribing-Newsletter.pdf
9 Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95.

10 Sivem [rabeprazole monograph; content updated 2014 Nov 19]. In: Compendium of pharmaceuticals and specialties (CPS) [online resource]. Ottawa (ON): Canadian Pharmacists Association; 2016 [cited 2016 Aug 21]. Available from: http://www.e-therapeutics.ca. Subscription required to access content.
11 Mössner J. The indications, applications, and risks of proton pump inhibitors. Dtsch Arztebl Int. 2016;113(27–28):447–83.
12 Targownik L. Chapter 60: Dyspepsia and peptic ulcer disease. In: Compendium of therapeutic choices. 7th ed. Ottawa (ON): Canadian Pharmacists Association; 2014. p. 733–43.
13 Sun J, Yuan YZ, Hou XH, Zou DW, Lu B, Chen MH, et al. Esomeprazole regimens for reflux symptoms in Chinese patients with chronic gastritis. World J Gastroenterol. 2015;21(22):6985–73.
14 Iwakiri K, Kinoshita Y, Habu Y, Oshima T, Manabe N, Fujiwara Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol. 2016;51(8):751–67.
15 Residential Care Advisory Committee of the College of Pharmacists of British Columbia. Interpretation manual for residential care facilities and homes: standards of practice. Vancouver (BC): College of Pharmacists of British Columbia; 2011 Jan [cited 2016 Aug 1]. 66 p. Available from: http://library.bcpharmacists.org/H-Resources/H-4_Pharmacy_Resources/5097-Interpretation_Manual_Residential_Care.pdf
16 Keung C, Hebbard G. The management of gastro-oesophageal reflux disease. Aust Prescr. 2016;39(1):6–10.

17 Schiff GD, Seoane-Vazquez E, Wright A. Incorporating indications into medication ordering-time to enter the age of reason. N Engl J Med. 2016;375(4):306–9.
18 Björnsson E, Abrahamsson H, Simrén M, Mattsson N, Jensen C, Agerforz P, et al. Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;24(6):945–54.
19 Suzuki H, Okada S, Hibi T. Proton-pump inhibitors for the treatment of functional dyspepsia. Ther Adv Gastroenterol. 2011;4(4):219–26.
Competing interests: Aaron M Tejani has received payment for expert testimony and honoraria from various groups and organizations for speaking engagements and presentations unrelated to the work presented here. No other competing interests were declared. ( Return to Text )
Address correspondence to: Dr Aaron M Tejani, LMPS Vancouver Office, 2733 Heather Street, Heather Pavilion, Level D (4th Floor), Vancouver BC V5Z 1M9, e-mail:aaron.tejani@ti.ubc.ca
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 71, NUMBER 5, September-October 2018