Jason Yung, Tiffany Nguyen, Robert MacLean, Jason WentzellABSTRACT
Background
The layered learning practice model (LLPM), within which a pharmacist supervises both a pharmacy resident and a student, mitigates the growing demand for clinical rotations that has accompanied national expansion of Doctor of Pharmacy programs. A Canadian collaborative of hospital pharmacists established consensus on 8 clinical pharmacy key performance indicators (cpKPIs), activities associated with improved patient outcomes. Increased implementation of the LLPM alongside cpKPI measurement offers opportunities to compare the LLPM with standard practice in terms of pharmaceutical care delivery.
Objective
To quantify clinical productivity, as measured by proportions of eligible patients receiving cpKPIs and absolute numbers of completed cpKPIs, across scenarios involving pharmacists working with and without pharmacy learners.
Methods
In this retrospective observational study, pharmacy students, pharmacy residents, and pharmacists recorded completion of 7 cpKPIs for oncology inpatients over a total of 6 months in 2017 and 2018. Clinical productivity was described across the following 3 scenarios: presence of one or more pharmacists with one resident and one or more students (P-R-S); presence of one or more pharmacists with one or more students (P-S); and presence of one or more pharmacists only (P; standard practice).
Results
During the study, there were 685 recorded admissions to the inpatient oncology service. Generally, the proportions of patients who received cpKPIs were similar for scenarios with and without pharmacy learners present. Standardized to 20 pharmacist workdays, the total number of cpKPIs 1, 2, 3, 5, 6, and 7 (255 with P-R-S scenario, 281 with P-S scenario, and 258 with P scenario) and the total number of drug therapy problems resolved (i.e., cpKPI 3; 153 with P-R-S scenario, 180 with P-S scenario, and 149 with P scenario) were similar across the scenarios. Scenario P had fewer admitted patients per pharmacist workday (3.2) than scenarios P-S and P-R-S (3.4 and 3.7, respectively), which may have contributed to a trend toward greater proportions of patients receiving cpKPIs under scenario P.
Conclusions
Compared with standard practice, integration of pharmacy learners within an oncology unit did not appear to impair clinical productivity, as demonstrated by the comparable proportions of patients receiving cpKPIs and the total number of completed cpKPIs.
KEYWORDS: clinical pharmacy key performance indicators, layered learning practice model, hospital pharmacy, pharmacy learner, clinical productivity
RÉSUMÉ
Contexte
Le modèle de pratique avec apprentissage à plusieurs niveaux (traduction libre de : Layered Learning Practice Model, [LLPM]), où un pharmacien supervise un résident et un étudiant en pharmacie, permet de réduire la demande croissante de stages cliniques qui a suivi le développement national des programmes de doctorat en pharmacie. Un regroupement canadien composé de pharmaciens d’hôpitaux a établi un consensus sur huit indicateurs clés de rendement relatifs à la pharmacie clinique (ICRpc), activités associées à l’amélioration des résultats thérapeutiques. L’accélération de la mise en oeuvre du LLPM, parallèlement à l’évaluation des ICRpc, offre des occasions de comparer le LLPM aux pratiques courantes en ce qui a trait à la prestation de soins pharmaceutiques.
Objectif
Quantifier la productivité clinique, en fonction des proportions de patients admissibles, profitant des ICRpc et des nombres absolus d’ICRpc évalués, dans des scénarios où les pharmaciens travaillent ou non avec des étudiants ou des résidents.
Méthodes
Dans la présente étude d’observation rétrospective, des étudiants et des résidents en pharmacie ainsi que des pharmaciens ont enregistré l’évaluation complète de sept ICRpc pour des patients hospitalisés en oncologie sur une durée totale de six mois en 2017 et 2018. La productivité clinique a été décrite à l’intérieur des trois scénarios suivants : participation d’au moins un pharmacien accompagné d’au moins un résident et un étudiant (P-R-É); participation d’au moins un pharmacien accompagné d’au moins un étudiant (P-É); et participation d’au moins un pharmacien, sans étudiant ou résident (P : pratique courante).
Résultats
Au cours de l’étude, on a enregistré 685 admissions au service d’hospitalisation en oncologie. Généralement, les proportions de patients profitant des ICRpc étaient semblables dans les trois scénarios. Basé sur une unité de mesure de 20 jours de travail de pharmacien, le nombre total d’ICRpc 1, 2, 3, 5, 6 et 7 (255 pour le scénario P-R-É, 281 pour le scénario P-É et 258 pour le scénario P) et le nombre total de problèmes pharmacothérapeutiques réglés (c’est-à-dire ICRpc 3; 153 pour le scénario P-R-É, 180 pour le scénario P-É et 149 pour le scénario P) étaient semblables dans les différents scénarios. Le scénario P présentait moins de patients admis par jours de travail de pharmacien (3,2) que les scénarios P-É et P-R-É (respectivement 3,4 et 3,7), ce qui peut avoir contribué à créer une tendance montrant une plus grande proportion de patients profitant des ICRpc dans le scénario P.
Conclusions
Comparée à la pratique courante, l’intégration d’étudiants ou de résidents en pharmacie dans un service d’oncologie ne semblait pas réduire la productivité clinique, comme l’illustrent les proportions comparables de patients profitant d’ICRpc et le nombre total d’ICRpc évalués.
MOTS CLÉS: indicateurs clés de rendement relatifs à la pharmacie clinique, layered learning practice model, pharmacie hospitalière, étudiant en pharmacie, productivité clinique
Health care–related key performance indicators (KPIs) are quantifiable measures of quality that may be used to track an organization’s performance in specific critical processes and outcomes.1 KPIs have been shown to be associated with positive patient outcomes.2 The measurement of KPIs contrasts with workload metrics—the frequencies at which various activities are performed—which are not necessarily correlated with patient outcomes.2 By extension, a clinical pharmacy KPI (cpKPI) is a standardized quantitative measure of progress for a specific clinical activity performed by a pharmacist.1 As such, cpKPIs serve as objective indicators by which to measure the efficiency of delivery of evidence-based patient care processes.3
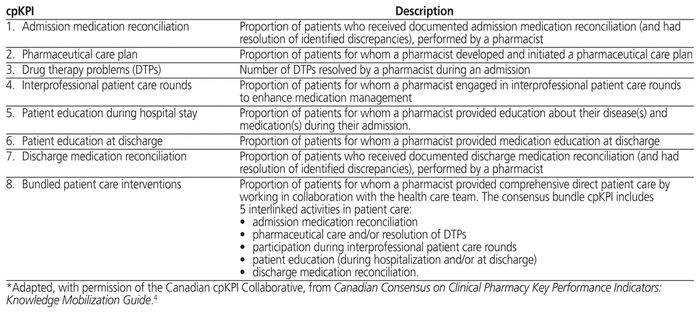
In 2013, a Canadian collaborative of clinical pharmacists and hospital pharmacy leaders established consensus on 8 national cpKPIs representing essential patient care processes.4 These 8 cpKPIs (Table 1) relate to aspects of an admitted patient’s hospital course and are associated with evidence-informed improvements in meaningful patient outcomes.4 For instance, it has been shown that inpatient team–based pharmacists who perform proactive patient care activities, such as conducting admission medication reconciliation and resolving drug therapy problems (DTPs), significantly reduce the number of hospital readmissions and patient mortality.1,5,6 By reporting the value of clinical pharmacy services through quantification of cpKPIs, hospital administrators have standardized metrics that may support the maintenance or expansion of clinical pharmacy services to provide evidence-based care.2
Table 1 Canadian Consensus Clinical Pharmacy Key Performance Indicators (cpKPIs)*
With the expansion of entry-to-practice Doctor of Pharmacy (PharmD) and PharmD Bridging programs across Canada, there has been an increase in the demand for clinical experiential rotations that pharmacy learners must complete.7 To accommodate a larger number of learners and to meet the increasing demands of the health care system, practice sites have implemented the layered learning practice model (LLPM).8–10
Within the LLPM framework, pharmacy learners at different levels of training (i.e., pharmacy students, pharmacy residents) provide patient care under the guidance of a pharmacist preceptor.8,9,11 Delgado and others11 found that this model enabled pharmacy students to effectively act as “pharmacist extenders”, providing comprehensive pharmacy services to patients who would otherwise not be reached. This model also facilitates near-peer teaching among learners, whereby senior peers provide learning support to junior students, drawing on their comparable knowledge base.7 It offers students access to more learning opportunities without unduly increasing the pharmacist’s workload, and enables residents to hone their skills as preceptors through mentoring of pharmacy students within a supervised structure.7,12 In a qualitative study, Bates and others13 assessed the delivery of experiential education to Advanced Pharmacy Practice Experience students and pharmacy residents in an oncology LLPM environment. They found that the LLPM framework was well perceived by learners and did not compromise the achievement of knowledge-based learning objectives.
Chow and others14 evaluated whether there was a difference in the number of patients who received admission medication reconciliation (one of the Canadian consensus cpKPIs) between learner–pharmacist pairs and pharmacists alone. The authors of this 6-month study concluded that the number of admission medication reconciliations completed per 5-week rotation increased by a median of 5 when a pharmacy learner was present.14 In another study, Bates and others9 described the frequency at which patients in malignant hematology and medical oncology services received discharge medication reconciliation and counselling in an LLPM. They observed that with this model, 51% of all patients received personalized education upon discharge from the pharmacy team, compared with 0% of patients before the study.9 Accordingly, the authors reported that the integration of pharmacy learners into an LLPM expanded the provision of pharmacist services.9
There is a lack of literature describing and quantifying clinical productivity in the LLPM, and an even greater paucity of literature quantifying the contributions of pharmacy learners to patient care. More specifically, no published studies have evaluated the delivery of all 8 cpKPIs in the presence of pharmacy learners. In this pilot study, we aimed to bridge these gaps in the literature by capturing data for all of the cpKPIs recommended by the Canadian cpKPI Collaborative and by quantifying the delivery of patient care services by different combinations of pharmacy professionals across a spectrum of the LLPM. We evaluated clinical productivity, as measured by the completion of cpKPIs by pharmacists working in the presence or absence of pharmacy learners (students with or without residents).
This study involved the following 3 scenarios under an inpatient medical oncology service of a tertiary care centre at different times during the study timeframe: presence of one or more pharmacists with one resident and one or more students (P-R-S); presence of one or more pharmacists with one or more students (P-S); and presence of one or more pharmacists only (P; standard practice).
The primary objectives were to describe the proportions of patients who received cpKPIs 1 through 7 across the aforementioned scenarios under an inpatient medical oncology service; to determine the contributions of each respective pharmacy professional for each cpKPI across the aforementioned scenarios under the same inpatient service; and to describe the number of cpKPIs performed per pharmacy professional, standardized to 20 pharmacist workdays, across the aforementioned scenarios under the same inpatient service. The secondary objectives were to compare the number of DTPs resolved by each pharmacy professional, standardized to 20 pharmacist workdays, across the aforementioned scenarios under the medical oncology service, and to determine the proportion of eligible patients who received bundled patient care interventions (i.e., cpKPI 8) across the aforementioned scenarios under the same inpatient service.
We conducted a retrospective observational pilot study under the inpatient medical oncology service at a single tertiary care teaching centre. The inpatient medical oncology service is an interdisciplinary team that provides care to patients with acute, often complex health care needs. Patients admitted to the inpatient medical oncology service include those with acute infections, thromboembolism, cancer- or chemotherapy-related complications, or symptoms of the underlying malignancy, and those needing disposition planning and palliation. The service is staffed with 2 full-time equivalent pharmacists who generally work 7.5-h workdays from Monday to Friday, with occasional weekends. About 65% of each pharmacist’s time is dedicated to the provision of direct patient care services, which includes clinical activities defined by the cpKPIs. About 25% of each pharmacist’s time is devoted to centralized pharmacy distribution tasks, including verification of chemotherapy orders and screening of medication orders for hospitalized inpatients. The remaining (estimated) 10% of time is directed toward administrative, educational, research, or quality improvement-based initiatives. Preceptorship of pharmacy learners within this practice framework is generally performed within the time allotted for provision of clinical services, although departmental efforts are made to help alleviate some distribution service requirements for pharmacists when they are working as preceptors with assigned students.
A 6-month convenience period was established and served as a feasible timeframe during which multiple pharmacy learners (residents and students) had planned direct patient care oncology rotations. During the study, 4 medical oncology pharmacists (total of 2 full-time equivalent positions), 2 pharmacy residents, and 5 PharmD students (4 fourth-year pharmacy students and 1 post-bachelor PharmD Bridging student) were involved in providing care on the medical oncology service. Appendix 1 (available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/190/showToc) provides the specific dates and durations of the respective rotations and a description of scheduling overlap.
All pharmacy professionals (pharmacists, pharmacy residents, and pharmacy students) received both standardized instruction and a copy of the project manual (Appendix 2, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/190/showToc). The project manual classified each cpKPI and provided examples of the various DTPs, categorized from A to G (as listed in Addendum B of Appendix 2). Each participant was also given a package of customized printed stickers, denoting each of the 7 standardized cpKPIs. Pharmacists were given white stickers, pharmacy residents were given pink stickers, and pharmacy students received yellow stickers. Upon performing a particular cpKPI, the individual was instructed to affix the appropriate sticker onto his or her own daily patient care roster (Addendum C of Appendix 2). Certain of the cpKPIs required additional documentation (Table 2). For example, participants were instructed to track the number and types of DTPs identified and resolved by documenting a letter (A to G) on the labels, which were assigned to specific DTPs. No patient-specific data were collected.
Table 2 Additional Sticker Documentation Requirements for Tracking Clinical Pharmacy Key Performance Indicators (cpKPIs) on Patient Care Rosters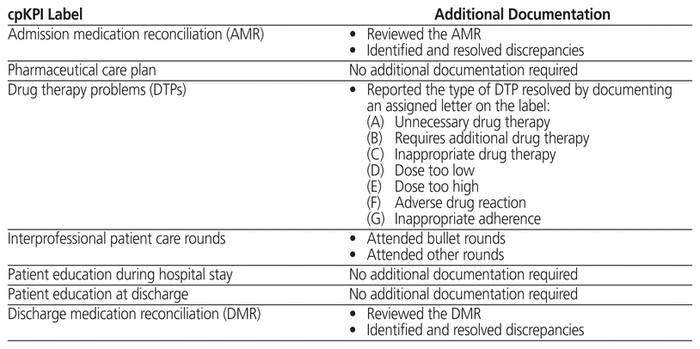
Before the study began, there was a 2-week lead-in period (March 27 to April 7, 2017), during which participants recorded completion of cpKPIs, to become familiar with the cpKPI documentation procedures. The aim of the lead-in period was to equip pharmacist preceptors with the knowledge and skills to ensure appropriate recording of cpKPIs for themselves and their pharmacy learners. The lead-in period was applied only for the first group of participants; subsequent pharmacy professionals who contributed to data collection received standardized instruction and the project manual (described above). Data recorded during the lead-in period were not included in data analyses.
Completed cpKPIs were recorded for all patients admitted under the medical oncology service at the hospital during the periods April 10 to September 15, 2017, and January 8 to February 9, 2018 (for a total study timeframe of 6 months). Patients who did not receive any pharmacy services that would warrant recording of a cpKPI were included, to ensure accurate estimation of proportions of patients receiving the respective cpKPIs. Patients who died during their admission were not eligible to receive cpKPI 6 (education at discharge), cpKPI 7 (discharge medication reconciliation), or the bundled patient care intervention, and were excluded from these assessments. Whenever a pharmacy learner was present under the oncology service, debriefing sessions occurred daily. During these meetings, the oncology pharmacist(s) and the pharmacy learner(s) reviewed respective patient care plans and discussed the clinical activities that had been performed during the day. This process encouraged standardized documentation and facilitated appropriate assignment of cpKPIs among participants who may have provided pharmaceutical care to the same patients.
During the study, patient care rosters with the affixed stickers were collected weekly and stored in a secure area within the pharmacy. A de-identified, password-protected quality assurance database was created to electronically record the number and timing of completed cpKPIs. The recorded data from patient rosters were entered into the electronic database by a PharmD student and were validated by the primary investigator (J.W.). Approval for this study was granted by the institutional Research Ethics Board.
Given the exploratory nature of this study, descriptive statistics were used to report the study outcomes. As recommended by the Canadian cpKPI Collaborative,4 data for cpKPIs 1, 2, 4, 5, 6, and 7 are reported as proportions of patients receiving the cpKPIs, and data for cpKPI 3 are reported as total number of DTPs resolved. As an additional aspect of cpKPI 3, the proportion of patients with DTPs resolved is also reported. The number of 7.5-h pharmacist workdays was determined by summing the total number of pharmacist working days during the respective intervention periods, which serves to account for differences in staffing or vacation that occurred between periods. The number of cpKPIs performed within each scenario was then adjusted to 20 pharmacist workdays to demonstrate the volume of respective cpKPIs completed per pharmacist over a period approximating 1 month of clinical service. In addition, we report on each pharmacy professional’s contributions to the total proportions of patients receiving the various cpKPIs and the number of cpKPIs standardized to 20 pharmacist workdays. Because this was a descriptive study, no formal statistical analyses were performed.
In total, 685 recorded admissions to the hospital’s medical oncology service occurred over the 6 months of the study (April 10 to September 15, 2017, and January 8 to February 9, 2018). The number of admitted patients per pharmacist workday, a surrogate marker of pharmacists’ workload, was lower for the pharmacist-only scenario (3.2) than for the P-S and P-R-S scenarios (3.4 and 3.7, respectively) (Table 3).
Table 3 Baseline Characteristics across the 3 Scenarios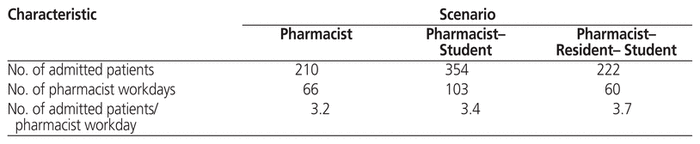
Figure 1 depicts the total proportions of eligible patients who received the various cpKPIs, as well as contributions to patient care from each pharmacy professional within each of the scenarios. Despite a consistent trend for pharmacists to contribute less to overall patient care when learners were present, more so when both a pharmacy resident and one or more students were present, the total proportions of patients receiving cpKPIs appeared generally similar across all scenarios. Furthermore, there may have been a trend toward higher proportions of patients receiving cpKPIs in the pharmacist-only scenario, compared with scenarios in which pharmacy learners were present. Scenario P was also noted to have a greater proportion of eligible patients who received bundled patient care interventions, relative to scenarios P-S and P-R-S (Figure 2). These findings may be attributable to the fact that the pharmacist-only scenario had full staffing, with no vacation, and also had the smallest relative workload, as represented by the number of admitted patients per pharmacist workday, compared with scenarios in which pharmacy learners were present (Table 3).
|
|
||
|
Figure 1 Proportions of eligible patients who received clinical pharmacy key performance indicators (cpKPIs) within each scenario. All patients (100%) received interprofessional patient care rounds in all 3 scenarios (where P = pharmacist present; P-S = pharmacist and student present; and P-R-S = pharmacist, resident, and student present). Abbreviations for cpKPIs: AMR = admission medication reconciliation, PhCP = pharmaceutical care plan, DTPs = drug therapy problems, EduHosp = education during hospitalization, EduDisch = education during discharge, DMR = discharge medication reconciliation. |
||
|
|
||
|
Figure 2 Proportions of eligible patients who received bundled patient care interventions within each scenario. |
||
The largest identified discrepancy in care delivery occurred for cpKPI 7, discharge medication reconciliation. Within the LLPM model investigated here, daily pharmacist and learner debriefings occurred in the afternoon, the time of day when many patients are discharged; this could explain, in part, the difference in completion of cpKPI 7 among different scenarios.
At least one member of the clinical pharmacy team contributed to patient care through attending and participating in the daily interdisciplinary discharge rounds (cpKPI 4). Because of this consistent attendance at rounds, all patients within the study were deemed to have received cpKPI 4 throughout their respective admissions, and there were no differences among the scenarios.
The total number of cpKPIs 1, 2, 3, 5, 6, and 7, standardized to 20 pharmacist workdays, was similar across scenarios (255 with the P-R-S scenario, 281 with the P-S scenario, and 258 with the P scenario) (Figures 3 and 4). We also observed a potential trend toward resolution of more DTPs with pharmacy learners present (153 with the P-R-S scenario, 180 with the P-S scenario, and 149 with the P scenario) (Figure 4). The most common DTP resolved across all scenarios was initiation of medications for patients (reported as “additional drug” in Figure 4), which included chemotherapy and associated supportive care medications, such as antiemetics. The second most commonly resolved DTP across all scenarios was discontinuation of a medication because a clinical indication was lacking. The absolute increase in DTPs identified when learners were present may be attributable to the comprehensiveness of learners’ respective care plans and their thorough review of medications.
|
|
||
|
Figure 3 Number of clinical pharmacy key performance indicators (i.e., cpKPIs 1, 2, 5, 6, and 7) performed, with standardization to 20 pharmacist workdays. Scenario abbreviations: P = pharmacist present; P-S = pharmacist and student present; P-R-S = pharmacist, resident, and student present. Abbreviations for cpKPIs: AMR = admission medication reconciliation, PhCP = pharmaceutical care plan, EduHosp = education during hospitalization, EduDisch = education during discharge, DMR = discharge medication reconciliation. |
||
|
|
||
|
Figure 4 Number of drug therapy problems (DTPs) resolved, standardized to 20 pharmacist workdays. Scenario abbreviations: P = pharmacist present; P-S = pharmacist and student present; P-R-S = pharmacist, resident, and student present. |
||
In this study—which to our knowledge is the most comprehensive of its type to date—the pharmacist-only scenario had a lower number of admitted patients per pharmacist workday than the scenarios with pharmacy learners present. This difference in workload may have affected the results displayed in Figure 1, which appears to show a slightly greater proportion of patients receiving the various cpKPIs under scenario P than under scenarios P-S and P-R-S. In practical terms, clinical productivity did not appear to be impaired with the integration of pharmacy learners on the medical oncology team. Despite the progressive reductions in pharmacists’ contributions to completed cpKPIs in the presence of pharmacy students and a pharmacy resident, the proportions of patients receiving cpKPIs were largely comparable across all scenarios. This result is emphasized by the fact that the absolute total numbers of completed cpKPIs were largely consistent across the 3 scenarios. For this study, describing completed cpKPIs in an absolute fashion is important to demonstrate the maintenance and consistency of clinical productivity with learners present. This approach contrasts with reporting completed cpKPI proportions alone, which may be influenced by overall pharmacist staffing and patient volume across the respective scenarios.
Providing orientation, instruction, teaching, and mentorship to learners requires time that might otherwise be directed to clinical activities, which might in turn raise concerns about potential detriments to patient care. However, this study has shown that clinical work does not have to be neglected when learners are present. Rather, pharmacy activities can be appropriately delegated to, and completed by, pharmacy learners, thereby maintaining clinical productivity within an LLPM. A next logical avenue of research would be to explore rotational structures and strategies to improve clinical productivity within an LLPM. The provision of standardized, reproducible training and orientation, consistent definition of the roles of pharmacy professionals, and delegation of specified clinical tasks are all areas of potential optimization that may help to increase clinical productivity during pharmacy learner rotations.
The mean patient length of stay is another possible confounding factor that might have influenced the proportions of patients receiving cpKPIs across the scenarios. Although length of stay was not reported or examined in this study, scenarios with patients admitted for a longer duration would be more likely to have a greater proportion of patients receiving cpKPIs and, by extension, bundled patient care interventions. This study also did not specifically address the timing of hospital discharge. Patients whose discharges occurred outside of standard clinical or rotation hours, including evenings, weekends, or holidays, likely did not receive cpKPI 6 (discharge medication education) or cpKPI 7 (discharge medication reconciliation).
Another limitation of this study was the reliance on consistent and standardized documentation of completed cpKPIs by participants. During their medical oncology rotation, pharmacy learners were expected to develop pharmaceutical care plans for new patients and to perform follow-up for previously assigned patients, among other patient care activities and responsibilities. However, at the time of this study, the study institution did not have a systematic method of electronically tracking completion of patient-specific cpKPIs or resolution of specific DTPs by pharmacy team members. The multiple competing interests of pharmacy learners and clinical pharmacists might have precluded reliable documentation of all cpKPIs performed, a duty that was secondary to the provision of patient care. By extension, another limitation of this pilot study was the lack of evaluation of interindividual variations in cpKPI reporting among clinical pharmacists and pharmacy learners. Furthermore, workload and clinical productivity were not compared between pharmacy professionals at the same level of training. Further research is encouraged to confirm and extend the findings of this pragmatic study.
Notably, our study results corroborate those of Chow and others,14 who derived data from an electronic health record that tracked completion of cpKPIs. Those authors investigated whether the presence of pharmacy learners partnering with pharmacists affected the delivery of admission medication reconciliation, relative to standard practice.14 When standardized to a 5-week period, the investigators noted that the presence of a pharmacy learner significantly increased the number of admission medication reconciliations performed, with a median increase of 5 (29 versus 24).14 In our study, if the number of admission medication reconciliations were to be standardized to 25 pharmacist workdays (equivalent to a 5-week work period), there would be a similar increase of 5.3 with the presence of one or more pharmacy students (38.6 versus 33.3).
Although there is an established body of pharmacy practice research showing the impact of pharmacist interventions on patient outcomes,5,15,16 it is unknown whether pharmacy learner–specific interventions also lead to positive clinical outcomes. Literature comparing the quality of pharmaceutical care interventions among final-year pharmacy students, pharmacy residents, and clinical pharmacists is lacking. It might reasonably be hypothesized that the quality of patient care initiatives by the aforementioned pharmacy learners would be similar to that of clinical pharmacists, given that their interventions are performed in a manner consistent with, and under the supervision of, a pharmacist. Pharmacy learners are commonly assigned fewer patients than would be assigned to fully qualified pharmacists, because of challenges related to the complexity of cases and the management of a larger workload. Having a lower number of assigned patients often allows learners to develop comprehensive pharmaceutical care plans and to execute detailed patient care processes. Further research is encouraged to determine whether pharmacy learners’ contributions to care are associated with improved patient outcomes.
Pharmacist preceptors were responsible for teaching foundational therapeutic knowledge, coaching pharmacy learners on particular activities (e.g., discharge patient education), and reviewing documentation performed by pharmacy learners. Despite these competing interests, the results of this study demonstrated that clinical productivity could be maintained while the pharmacist supervised final-year pharmacy students, with or without a pharmacy resident. There was a maximum of 3 pharmacy learners (all pharmacy students) during only one week of the entire study, with supervision by 2 pharmacists. Although not explicitly examined in our study, there may be a threshold number of pharmacy learners at which point clinical productivity declines because of increased devotion of pharmacist work hours to preceptor duties. Another consideration that may need to be accounted for is whether pharmacists are staying after work hours in order to maintain overall clinical productivity during periods of preceptorship. This study did not reliably record or quantify whether the pharmacist preceptors worked extra hours during periods when learners were present. Subsequent ongoing institutional research aims to address this question within a similar context.
Because of the timing of planned PharmD student rotations, this study did not specifically examine a period when the pharmacy resident served as the sole pharmacy learner under the supervision of a pharmacist preceptor. More research is required to identify strategies to optimize the role of the pharmacy resident, who acts as a preceptor to pharmacy students, to maximize clinical productivity within the LLPM. These strategies should also meet the accreditation standards and educational needs of pharmacy residents within their clinical rotations.
At a practical level, the integration of pharmacy learners within an inpatient medical oncology service did not appear to impair clinical productivity. Although pharmacist contributions to patient care were reduced when pharmacy learners were present, overall patient care activities were maintained through delegation of these activities to the pharmacy learners. This study showed that the collaboration between pharmacists and pharmacy learners in a spectrum of the LLPM allowed provision of cpKPIs to similar proportions of patients and delivered comparable total numbers of cpKPIs relative to standard practice. Research is currently ongoing to identify strategies to optimize clinical productivity within an LLPM, which may include designation of specific roles to pharmacy students and enhanced delegation of teaching opportunities to pharmacy residents. Further studies are required to determine whether there are benchmarks for the proportion and number of completed cpKPIs that would affect patient outcomes at a population level.
1 Fernandes O, Gorman SK, Slavik RS, Semchuk WM, Shalansky S, Bussières JF, et al. Development of clinical pharmacy key performance indicators for hospital pharmacists using a modified Delphi approach. Ann Pharmacother. 2015;49(6):656–69.
2 Lo E, Rainkie D, Semchuk WM, Gorman SK, Toombs K, Slavik RS, et al. Measurement of clinical pharmacy key performance indicators to focus and improve your hospital pharmacy practice. Can J Hosp Pharm. 2016; 69(2):149–55.

3 Gorman SK, Slavik RS. Should key performance indicators be a component of performance assessment for individual clinical pharmacists? The “pro” side. Can J Hosp Pharm. 2014;67(2):165–6.
4 Fernandes O, Toombs K, Pereira T, Lyder C, Bjelajac Mejia A, Shalansky S, et al. Canadian consensus on clinical pharmacy key performance indicators: knowledge mobilization guide. Ottawa (ON): Canadian Society of Hospital Pharmacists; 2015 [cited 2018 Jul 22]. Available from: https://www.cshp.ca/clinical-pharmacy-key-performance-indicators
5 Makowsky MJ, Koshman SL, Midodzi WK, Tsuyuki RT. Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study [NCT00351676]. Med Care. 2009;47(6):642–50.
6 Minard LV, Deal H, Harrison ME, Toombs K, Neville H, Meade A. Pharmacists’ perceptions of the barriers and facilitators to the implementation of clinical pharmacy key performance indicators. PLoS One. 2016;11(4): e0152903.

7 Leong C, Battistella M, Austin Z. Implementation of a near-peer teaching model in pharmacy education: experiences and challenges. Can J Hosp Pharm. 2012;65(5):394–8.

8 Raman-Wilms L. Marriage of individual pharmacists’ achievement on key performance indicators and teaching responsibilities [editorial]. Can J Hosp Pharm. 2014;67(2):978.
9 Bates JS, Buie LW, Amerine LB, Savage SW, Eckel SF, Patel R, et al. Expanding care through a layered learning practice model. Am J Health Syst Pharm. 2016;73(22):1869–75.
10 Cameron K, Fernandes O, Musing EL, Raymond C. Increasing capacity for experiential rotations for pharmacy learners: lessons learned from a multisite teaching hospital. Can J Hosp Pharm. 2016;69(1):23–9.

11 Delgado O, Kernan WP, Knoer SJ. Advancing the pharmacy practice model in a community teaching hospital by expanding student rotations. Am J Health Syst Pharm. 2014;71(21):1871–6.
12 Sin JH, Li H, Jandovitz N, King M, Tsapepas DS. Dynamic interplay of pharmacy learners during a solid organ transplantation learning experience. J Pharm Pract. 2018;31(3):347–52.
13 Bates JS, Buie LW, Lyons K, Rao K, Pinelli NR, McLaughlin JE, et al. A study of layered learning in oncology. Am J Pharm Educ. 2016;80(4): Article 68.
14 Chow M, Lui P, Romanko A, Cameron K, Hamandi B, Gorman SK, et al. Assessment of the impact of pharmacy learners on essential patient care processes [abstract]. Can J Hosp Pharm. 2017;70(1):58.
15 Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900.
16 Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27(4):481–93.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors would like to acknowledge and thank the following individuals for their contributions pertaining to this project: Jennifer Spencer, Uzo Onochie-Roy, and Stephanie Lovering served as oncology preceptors throughout the study, ensuring standardization of practical data recording and promoting familiarity with the project and process; Steven Lam and Andrew Osinga assisted the lead author (J.W.) with development of the initial institutional cpKPI manual, which served as a standardized teaching tool throughout the study; Jennifer Spencer, Uzo Onochie-Roy, Erin Francis, Melanie Trinacty, Darcy McLurg, Joy Rashid, and Stephanie Lovering served as protocol and study consultants leading to initial protocol development; and Amanda Owen was hired by the study institution to assist with data collection.
Canadian Journal of Hospital Pharmacy, VOLUME 72, NUMBER 3, May-June 2019