Emily K Black, Lindsay MacDonald, Heather L Neville, Kim Abbass, Kathryn Slayter, Lynn Johnston, Ingrid SketrisABSTRACT
Background
Antimicrobial use is the major factor in the development of antimicrobial resistance. Antimicrobial stewardship has been recommended as a strategy to improve antimicrobial use.
Objective
To learn about health care providers’ perceptions of current antimicrobial use and stewardship, including barriers and facilitators to improving antimicrobial use at acute care hospitals in Nova Scotia.
Methods
This qualitative research study was conducted at acute care hospitals in Nova Scotia using focus groups and semistructured interviews. Health care providers (nurses, nurse practitioners, pharmacists, pharmacy students, and physicians) were invited to participate. Focus groups and interviews were conducted at each participant’s place of employment. Interviews and focus groups were facilitated with an interview guide, audio-recorded, and transcribed verbatim. Transcripts were independently coded by 2 investigators and analyzed using thematic analysis.
Results
A total of 9 focus groups and 3 individual interviews were conducted between June and August 2017. Fifty-four health care professionals and trainees (24 pharmacists and pharmacy students, 14 physicians, and 16 nurses and nurse practitioners) from 5 hospitals participated. The following themes were identified: current practices, prescribing influences, access to information, collaboration and communication, resources, and antimicrobial stewardship. Within each theme, barriers and facilitators to improving antimicrobial use were identified as subthemes.
Conclusion
Participants identified current barriers to appropriate use of antimicrobials and suggested facilitators that might improve the use of these drugs. The results of this study could be used by antimicrobial stewardship teams and decision-makers to improve antimicrobial use and stewardship initiatives throughout Nova Scotia, and may be applicable to hospitals outside the province.
KEYWORDS: antimicrobial stewardship, infectious disease, antibiotic, antimicrobial
RÉSUMÉ
Contexte
L’utilisation des antimicrobiens est le principal facteur de développement de la résistance à cette classe de médicaments. La gestion des antimicrobiens a été recommandée comme stratégie visant à améliorer leur utilisation.
Objectif
Découvrir la perception des fournisseurs de soins de santé au sujet de l’utilisation et de la gestion actuelles des antimicrobiens, y compris les obstacles et les moyens destinés à favoriser l’amélioration de leur utilisation dans des hôpitaux de soins actifs en Nouvelle-Écosse.
Méthodes
Cette recherche qualitative a été menée dans des hôpitaux de soins actifs en Nouvelle-Écosse à l’aide de groupes de discussion et d’entretiens semi-structurés. Les fournisseurs de soins de santé (infirmières, infirmières praticiennes, pharmaciens, étudiants en pharmacie et médecins) ont été invités à y participer. Les groupes de discussion et les entretiens ont été menés sur chaque lieu de travail des participants. Ils ont été facilités grâce à un guide d’entretien. Ils ont aussi été enregistrés (audio) et retranscrits textuellement. Les transcriptions ont été codées de façon indépendante par deux enquêteurs et étudiées à l’aide d’une analyse thématique.
Résultats
Neuf groupes de discussion et trois entretiens individuels ont été menés entre juin et août 2017. Cinquante-quatre professionnels et stagiaires de la santé (24 pharmaciens et étudiants en pharmacie, 14 médecins, 16 infirmières et infirmières praticiennes) provenant de cinq hôpitaux y ont participé. Les thèmes suivants ont été soumis à la discussion : pratiques actuelles, influences en matière de prescription, accès aux informations, collaboration et communication, ressources et gestion des antimicrobiens. Chaque thème comportait deux sous-thèmes abordant les obstacles et les mesures favorisant l’amélioration de l’utilisation des antimicrobiens.
Conclusion
Les participants ont relevé les obstacles actuels nuisant à une bonne utilisation des antimicrobiens et ont proposé des moyens pour améliorer l’utilisation de ces médicaments. Les résultats de cette étude pourraient être utilisés par les équipes de gestion des antimicrobiens ainsi que par les décideurs qui doivent favoriser l’amélioration de l’utilisation des antimicrobiens et les initiatives relatives à leur gestion partout en Nouvelle-Écosse. Ils sont aussi applicables aux hôpitaux extérieurs à la province.
MOTS CLÉS: gestion des antimicrobiens, maladies infectieuses, antibiotiques, antimicrobiens
The international community has recognized antimicrobial resistance as a growing health concern.1 Without action, about 10 million deaths per year worldwide will be attributable to antimicrobial resistance by the year 2050.2 Infection with antimicrobial-resistant organisms has been associated with increased morbidity, mortality, cost, and burden to the health care system.3
Antimicrobial use is the major factor contributing to the development of antimicrobial resistance.4,5 Antimicrobial stewardship (AMS) has been suggested as a strategy to improve use of these drugs and is a core component of the pan-Canadian framework on tackling the problem of resistance.6,7 Successful AMS has resulted in several benefits, including reductions in antimicrobial use and associated costs, decreases in length of hospital stay, and improvements in adherence to prescribing policies.6,8
Despite the recognized value of AMS, further research is needed. In a recently published international consensus paper, identifying barriers and facilitators to implementing AMS programs and specifying activities in current programs were listed as priority areas requiring urgent scientific investigation to optimize AMS programs.9 Research on barriers and facilitators to implementation of interventions, with input from stakeholders on the design of programs, has also been recommended.8
A 2015 point prevalence survey of antimicrobial use in Nova Scotia identified targets for quality improvement.10 The objective of the qualitative study reported here was to explore the perceptions of stakeholders regarding barriers and facilitators to appropriate antimicrobial use and successful stewardship to guide effective implementation of AMS interventions.
A qualitative study was completed using focus groups and individual interviews for which a study-specific interview guide was developed and piloted before data collection began. Transcripts of both types of discussion were analyzed using thematic analysis. The study was part of a larger mixed-methods project and was informed by the results of the point prevalence survey.10 Potential participants were invited to attend a focus group or to complete an individual interview. The study was conducted in accordance with ethical standards of the responsible committees on human experimentation and the Helsinki declaration and was approved by the research ethics boards at the Nova Scotia Health Authority (file 100287) and the IWK Health Centre (file 1020269). Participants provided written informed consent.
The participants in focus groups and interviews were health care providers (nurses, nurse practitioners, pharmacists, and physicians) working at acute care hospitals in Nova Scotia. Pharmacy students were permitted to participate if they were completing a rotation with an invited participant. Health professionals were selected to receive an invitation on the basis of their role in direct care of patients with infectious diseases or their involvement and/or leadership in AMS initiatives at acute care hospitals in the province. Health care providers were purposely sampled from a range of specialities, specifically internal medicine, infectious diseases, infection control, surgery, pediatrics, emergency medicine, critical care, obstetrics, women’s and newborn health, and leadership/administration. Health care providers were invited verbally or through e-mail communication by site investigators at each hospital. Communication included information about the study objectives, study design, and expectations of participants. The e-mail invitation was sent to 122 individuals, and additional participants were invited verbally at one of the study sites. Participants were grouped by profession, with nurses, nurse practitioners, pharmacists, and pharmacy students participating jointly in focus groups, but separately from physicians. These groupings were based on feedback from the pilot phase, to increase participants’ willingness to contribute openly and to minimize perceived differences in authority.
All hospitals in Nova Scotia are part of the Nova Scotia Health Authority (NSHA), except the IWK Health Centre, which is a specialized hospital providing care to women, children, youth, and families.11 The study sites consisted of 2 specialized tertiary hospitals in large population centres and 3 regional hospitals in small to medium population centres. The sites were chosen to provide geographic representation from health care providers throughout the province. The IWK Health Centre established an AMS program in 2015, and the NSHA launched a provincial AMS program around the time of data collection in 2017 (http://www.cdha.nshealth.ca/nsha-antimicrobial-stewardship). All sites had treatment guidelines for select infectious syndromes. The IWK Health Centre launched an electronic application for disseminating guidelines a few months before data collection and provided prospective audit and feedback on antimicrobial prescribing for inpatients. At the time of data collection, the other sites were providing or were in the process of implementing prospective audit and feedback to select units within the health authority.
The focus groups and interviews took place at the participants’ respective places of employment using the study-specific interview guide, which was developed by members of the research team, who had expertise in infectious disease and pharmacotherapy, as well as experience with qualitative research. Additional feedback on the guide was sought from a qualitative researcher who was not otherwise involved in the study. The guide was piloted with 4 pharmacists and revised on the basis of feedback received. During each discussion, the moderator/interviewer provided participants with an overview of the research team, objectives of the current study, reasons for completing this project, and background on previously completed research evaluating anti-microbial use in Nova Scotia. Personal opinions and assumptions of the research team were not shared with participants. Focus groups and interviews were continued until representation from a diverse range of providers from different professions and specialities throughout Nova Scotia was obtained.
The focus groups and interviews were conducted between June and August 2017. All discussions took place in person, except for one telephone interview. Each focus group lasted about an hour, and the individual interviews lasted 30 minutes. Aside from the participants, only the principal investigator (E.B.) and a research assistant (L.M.) were present during discussions. Focus groups and interviews were moderated by the principal investigator, who maintains an active pharmacy practice licence, has completed a hospital pharmacy residency and Doctor of Pharmacy degree, holds an appointment as an Assistant Professor at Dalhousie University (with a research program focusing on antimicrobial use and stewardship), and provided about 100 h of clinical services with the infectious disease team at the Queen Elizabeth II Health Sciences Centre during the year before data collection. The research assistant was a pharmacy student who assisted by audio-recording the sessions and taking notes.
The data were interpreted using a thematic analysis. The analysis began during the interview or focus group process, with the interviewer/moderator asking probing questions as preliminary themes emerged. After each focus group or interview, the principal investigator and research assistant debriefed to discuss the emerging themes and perceived similarities and differences among interviewees and focus group participants. The research assistant then transcribed the recorded interviews verbatim. Each transcript was read in full by the principal investigator and the research assistant to better understand the discussion, followed by coding.12
Each transcript and related field notes were independently reviewed and coded in Microsoft Word (Microsoft Corporation, Redmond, Washington) by the 2 members of the research team (E.B., L.M.) who were present during interviews and focus groups. Coding was guided by a cyclic process, as described by Saldaña.13 The codes were initially determined with a process of open-coding, and emerging themes were noted thereafter. The principal investigator and research assistant each generated a code list, which included a description of code contents; they then compared and refined these 2 initial lists to create a unified codebook. The principal investigator functioned as the “codebook editor” and maintained the master list of codes.13 Transcripts were reviewed and independently recoded a minimum of 3 times by the principal investigator and research assistant. After each cycle of coding, the codes in the master codebook were further compared and refined. Before the final analysis was completed, the codebook was reviewed and revised by the full research team. After the third round of coding, a final list of themes and subthemes was prepared. During the final stage of analysis, codes were analyzed for similarities and differences by type of health care professional. Transcripts were not returned to participants for comment.
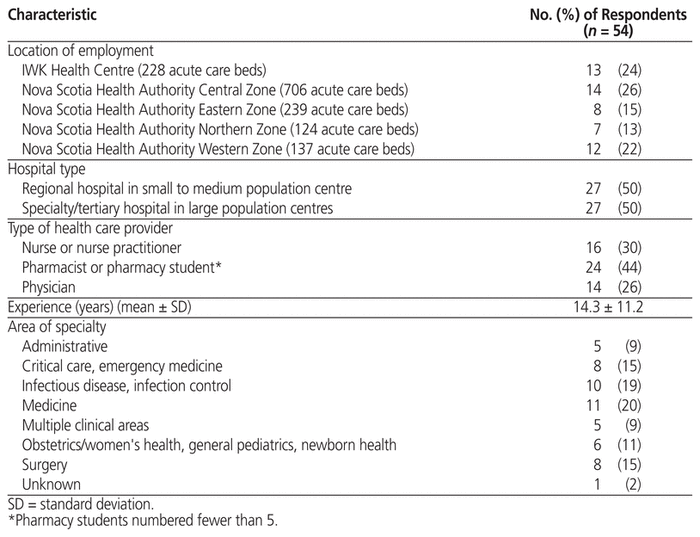
Nine focus groups and 3 interviews were completed with a total of 54 individuals, most of whom (n = 40) were unknown to the principal investigator; however, the principal investigator had a pre-existing relationship with 14 participants through undergraduate pharmacy training, committee involvement, clinical work, or research collaboration. All regions of Nova Scotia were represented. Baseline characteristics of participants are outlined in Table 1. Of those who did not participate, the proportions who were unavailable versus unwilling are unknown.
Table 1 Demographic Characteristics of Participants in Focus Groups and Interviews in a Study of Antimicrobial Stewardship in Nova Scotia Acute Care Hospitals
The following 6 themes were identified: current practices, prescribing influences, access to information, collaboration and communication, resources, and antimicrobial stewardship. For all of the themes, factors that affect antimicrobial use were identified (Figure 1). Within each theme, several individual-level and organizational or system-level barriers and facilitators to improve antimicrobial use were discussed by study participants. With the exception of one individual interview, each of the 6 themes was discussed in every focus group and interview. At sites where AMS initiatives were in place, discussions focused more extensively on facilitators and examples of success in improving antimicrobial use, whereas barriers and challenges formed the main topics of discussion at sites lacking those services and resources.
|
|
||
|
Figure 1 Themes and factors identified from health care providers’ perceptions of antimicrobial prescribing and use. |
||
Saturation of ideas occurred after 7 focus groups and 2 interviews. However, 2 additional focus groups and 1 additional interview were completed, to ensure geographic representation and inclusion of a diverse range of perceptions. Each theme is discussed below with representative quotations.
All groups recognized challenges with antimicrobial use. Suboptimal prescribing was most extensively discussed by focus groups involving pharmacists and nurses; however, physicians also acknowledged this challenge. Other challenges included inappropriate microbiological testing, antimicrobial resistance, and lengthy use of medical devices such as catheters and drains.
“The course of treatment. I can almost guarantee people overtreat everything in terms of course.” [Physician 36]
“That’s a full-time position right there to deal with the amount of urine cultures that go to the [microbiology laboratory] and identify whether they actually have symptoms or not.” [Nurse 22]
Specific barriers impeding improvement in antimicrobial use included prescribers’ resistance to recommendations from other health care providers at the individual level and lack of continuity of care at the organization/system level. Nurse/pharmacist focus groups primarily discussed prescriber resistance. Continuity of care, both in the hospital and upon discharge, was listed as a barrier across focus groups at the regional hospitals outside urban centres.
“Our patients are covered by multiple physicians that change over on certain days. They come on and say, ‘Well I’m just covering the weekend so you know, complete antibiotics until after the weekend.’ “ [Pharmacist 30]
Despite these barriers, the majority of participants reported increasingly judicious antimicrobial prescribing in recent years, and they were generally optimistic about future improvements in antimicrobial use.
“Over the last number of years we’ve seen them [prescribers] not jump to antibiotics right away. They seem to be rationalizing it a bit more.” [Pharmacist 3]
Participants shared a number of prescribing influences they perceived as affecting antimicrobial use. Knowledge, past experience, and external factors (such as patient pressure or perceptions of other health care providers) were influences that represented individual-level barriers or facilitators, depending on context.
“We’re seeing such diverse transition of physicians coming in, sometimes a locum … from out of province. They [prescribers] are nailing everybody with big guns because that is what they typically were used to.” [Nurse 11]
“I think sometimes what’s happening in a busy family practice is that you sort of succumb to the will of the parent demanding the antibiotics when maybe you don’t even feel like the patient actually needs it.” [Physician 52]
Diagnostic uncertainty, patient complexity, and prescriber fear were other individual-level barriers described in the study. Additionally, a few participants listed professional liability as both an individual-level and an organizational/system-level barrier that influenced prescribing. Other influences were patient-related factors, including clinical status, cost and convenience of the antimicrobial agent, antimicrobial characteristics, and local patterns of antimicrobial resistance.
“Empiric antibiotic use we’re comfortable with, but it’s the uncertainty when you’re unsure what infection you are treating. That’s one of the decisions around antimicrobial use or discontinuation that becomes the most challenging.” [Physician 51]
“In emerg [sic] I guess everyone’s just scared, so go broad just in case.” [Pharmacist 37]
Access to information was primarily discussed as a barrier at the organizational/system level. The most extensively discussed problem of this kind was lack of documentation in the health record, leading to challenges with inter- and intra-professional collaboration and extended duration of antimicrobial use. In contrast, some participants acknowledged that chart documentation in hospitals had improved recently.
“The indication not being included, it’s difficult for nursing and pharmacists to help, to be included in that therapy.” [Pharmacist 30]
Participants also reported challenges with accessing patient data, including current or previous antimicrobial use (at other institutions or in the community) and microbiology reports.
“So you have a patient coming from [another town] and they’ve had a nasopharyngeal swab for influenza. No one can access the results, so they send another one from here.” [Nurse 43]
Most participants indicated that these barriers need to be overcome to facilitate improvements in antimicrobial use.
Some participants listed inter- and intra-professional collaboration as an individual-level barrier to improving antimicrobial use. Conversely, other participants attributed success in this area to local collaboration among health care providers at their institution. Similarly, stakeholder involvement in developing policies or guidelines and community outreach were identified by some participants as a system-level barrier and by others as a facilitator, depending on their respective experiences.
“Whenever I’ve gotten a call from a pharmacist [to] bring to my awareness you know, IV [sic] or spectrum [of activity], I always appreciate it.” [Physician 33]
“To have a physician that will step in, physician-to-physician, and to have the conversation is huge.” [Nurse 20]
A few pharmacists also felt that a lack of confidence on the part of some health care providers to interact with physicians represented an individual-level barrier to improving antimicrobial use. One group discussed organizational complexity as a barrier that impeded efforts to improve antimicrobial use.
Lack of resources was identified as a barrier to improving the use of antimicrobials. Availability of adequate personnel and time were discussed extensively. Specific challenges with human resources included an inadequate number of health care providers with relevant expertise (infectious disease physicians, pharmacists, and AMS team members) and an inadequate number of experts to see the volume of patients requiring assessment. Some participants indicated that lack of time to contribute to initiatives in a busy clinic setting was a barrier. In contrast, a few groups indicated that access to dedicated AMS personnel or infectious disease specialists at their respective sites had facilitated successful implementation of AMS at the organizational level.
“Locally we just lost our infectious disease specialist … I think that really had a hit on our antimicrobial [use].” [Physician 24]
“I think we tried to pseudo-implement something before [our AMS pharmacist] showed up … but it was difficult. We didn’t have somebody specifically delegated and now, you have somebody you can go to and their attention isn’t divided to other things.” [Pharmacist 2]
Use of information technology was recognized as a facilitator that might address some barriers. Specific suggestions to improve access to information and prescribing included implementation of electronic medical records, physician order entry, computerized clinical decision support, and use of electronic applications (apps) to distribute clinical practice guidelines.
“I do find the peds [sic] residents, in particular, are very excited to have this app and they do use it regularly … That’s their go-to in terms of writing out their starting orders.” [Pharmacist 4]
Most AMS initiatives were identified as facilitators that might improve antimicrobial use. Participants indicated a desire for prescribing supports, including implementation of guidelines and provision of enabling AMS interventions such as audit and feedback. Education and training for a variety of audiences, including health care providers and the general public, were also viewed favourably. Current gaps in knowledge and guideline uptake were listed as ongoing barriers that slowed progress in improving antimicrobial use.
“Guidelines, having our own-specific guidelines [for the institution] and now the app, it’s just huge … even I’m finding myself changing therapy that is appropriate to slightly more appropriate.” [Pharmacist 1]
“When you went to nursing school one of the first things they taught you was if the patient is confused or if their urine smelled or was cloudy you should send that, when that’s really not the case now in 2017 so I think it’s a lot of education for nursing staff as well.” [Nurse 22]
Other system-level facilitators that were identified included research, surveillance of antimicrobial use, antimicrobial resistance, monitoring and reporting on the impact of AMS interventions, and the size of the facility. At the individual level, personal attributes of health care providers, including expertise, respect, and collegiality in delivering initiatives, were recognized as facilitators of success.
“Having someone who’s pleasant and friendly and engages with other people really well and in a non-threatening, nonconfrontational way. I think that’s really critical when you’re trying to convince people to change practice.” [Physician 51]
Health care providers’ perceptions of restrictive interventions, including pre-authorization, were variable. Several participants indicated that previous attempts to implement restrictive interventions had led to poor relationships and “policing” of antimicrobial prescribing. Other participants felt that restrictive interventions would improve prescribing if delivered properly. Additional standardization of services was identified as a current system-level barrier that needed to be addressed to facilitate delivery of initiatives and improve antimicrobial use.
“We used to have restrictions and we got rid of them because it required a lot of policing [on] pharmacy’s part.” [Pharmacist 10]
Health care providers who participated in this study provided insights into antimicrobial use and stewardship in Nova Scotia. Barriers to and facilitators of improvements in antimicrobial use were underlying subthemes that crossed all major themes discussed. Participants recognized a number of modifiable barriers that need to be addressed to improve antimicrobial use. Despite these challenges, many participants indicated that progress had already been made in improving antimicrobial use. The findings from this study will be shared with members of AMS teams in Nova Scotia and used to inform more research on developing and implementing AMS interventions.
Although many of the barriers and facilitators identified in this study were consistent with those reported in the literature, 14–21 our findings contribute in several ways to a growing body of knowledge on AMS. Previously published qualitative studies were primarily completed in countries outside Canada, under different health care systems.14–19,21 In addition, few studies considered the perceptions of stakeholders from interdisciplinary teams in rural settings.18,19,21 A Canadian study by Pasay and others,20 which evaluated the perceptions of pharmacy staff about AMS policies and resources, highlighted the lack of generalizability to other professions. An Australian study by Bishop and others21 concluded that centralized organizations (as in Nova Scotia) should have a good understanding of local context, given specific considerations identified in regional hospitals. Consistent with recently published priorities for AMS research,9 our study adds to the literature by reporting the barriers and facilitators identified by an interdisciplinary group of stakeholders working in rural and urban publicly funded hospitals in a Canadian context.
Successful implementation of AMS interventions has been attributed to adequate resources and infrastructure, in addition to the establishment of relationships, with strong communication between AMS programs and clinical teams.22 However, one of the most widely discussed barriers in this study was lack of adequate personnel with expertise in infectious disease and/or AMS. To overcome this barrier, additional personnel with dedicated time to deliver interventions and increased access to infectious disease specialists, particularly in regional hospitals, are needed. Financial constraints may hinder attempts to increase personnel. Solutions for overcoming this barrier in resource-limited settings include providing further training for the current workforce, streamlining processes to reallocate clinician time to focus on AMS-related interventions, and use of technology such as telehealth. Incorporating these suggestions may capitalize on appropriate use of human resources.
Implementation of AMS was generally viewed as a facilitator. Consistent with our findings, specific interventions viewed favourably in previously published qualitative studies include enabling interventions such as audit and feedback delivered in a safe learning environment by content experts,16 educational initiatives,15,17 and guideline implementation.15,18 Restrictive interventions were listed as a barrier by some participants in our study and as a facilitator by others. Although restrictive interventions have shown benefit in terms of adherence to policies, they may lead to challenges in communication and trust between health care providers and can result in a delay in treatment.8 If restrictive interventions are being implemented, careful consideration of methods to ensure best delivery is suggested.
This study had several limitations. A pharmacist conducted the interviews and moderated the focus groups, which may have influenced participant response. In addition, the pharmacist who moderated the interviews had a pre-existing relationship with some of the participants. However, excluding participants known to the research team might have led to bias and poor validity through the omission of views of key stakeholders. We do not believe that facilitation of interviews by the principal investigator significantly affected the results, as similar themes were discussed across all groups. The funding available for this study did not support hiring additional research personnel with experience in qualitative research to facilitate the interviews and focus groups or to analyze the transcripts; however, members of our team, including the principal investigator, have previous experience in completing qualitative studies. Finally, results from this study represent the opinions of the study participants and may not be generalizable to other groups of health care providers or health care providers in other provinces. Despite these limitations, the themes discussed were relatively consistent across groups and likely represent accurately the perceptions of antimicrobial use and stewardship in Nova Scotia hospitals.
Findings from this study provide evidence of targets for improvement in a Canadian context, in both urban and rural publicly funded inpatient settings. Although the study identified numerous challenges in antimicrobial use, optimism was apparent. Modifiable barriers should be addressed to optimize the impact of AMS initiatives.
1 At UN, global leaders commit to act on antimicrobial resistance [news release]. Geneva (CH): World Health Organization; 2016 [cited 2018 Jul 7]. Available from: www.who.int/news-room/detail/21-09-2016-atun-global-leaders-commit-to-act-on-antimicrobial-resistance
2 Review on Antimicrobial Resistance; O’Neill J [chair]. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. London (UK): HM Government; 2014 [cited 2019 Jul 8]. Available from: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
3 Antibiotic resistance threats in the United States, 2013. Atlanta (GA): US Department of Health and Human Services, Centers for Disease Control and Prevention; 2013 [cited 2019 Jul 5]. Available from: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
4 Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:1–25.
5 Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6 Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Disease Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e5–77.
7 Tackling antimicrobial resistance and antimicrobial use: a pan-Canadian framework for action. Ottawa (ON): Public Health Agency of Canada; 2017 [cited 2019 Jul 5]. Available from: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/tackling-antimicrobialresistance-use-pan-canadian-framework-action.html
8 Davey P, Marwick CA, Scott CL, Charani E, McNeil K, Brown E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017;2:CD003543.

9 Rzewuska M, Charani E, Clarkson JE, Davey PG, Duncan EM, Francis JJ, et al. Prioritizing research areas for antibiotic stewardship programmes in hospitals: a behavioural perspective consensus paper. Clin Microbiol. 2019;25(2):163–8.
10 Black E, Neville H, Losier M, Harrison M, Abbass K, Slayter K, et al. Antimicrobial use at acute care hospitals in Nova Scotia: a point prevalence survey. Can J Hosp Pharm. 2018:71(4):234–42.

11 About us [webpage]. Halifax (NS): IWK Health Centre; [cited 2018 Jun 16]. Available from: http://www.iwk.nshealth.ca/page/about-us
12 Krueger RA, Casey MA. Focus groups: a practical guide for applied research. 5th ed. Thousand Oaks (CA): Sage Publications; 2014.
13 Saldaña J. The coding manual for qualitative researchers. Los Angeles (CA): Sage Publications; 2009.
14 Charani E, Castro-Sanchez E, Sevdalis N, Kyratsis Y, Drumright L, Shah N, et al. Understanding the determinants of antimicrobial prescribing within hospitals: the role of “prescribing etiquette”. Clin Infect Dis. 2013; 57(2):188–96.

15 Black E, Cartwright A, Bakharaiba S, Al-Mekaty E, Alsahan D. A qualitative study of pharmacists’ perceptions of, and recommendations for improvement of antibiotic use in Qatar. Int J Clin Pharm. 2014;36(4):787–94.
16 Cotta MO, Robertson MS, Marshall C, Thursky KA, Liew D, Buising KL. Implementing antimicrobial stewardship in the Australian private hospital system: a qualitative study. Aust Health Rev. 2015;39(3):315–22.
17 Giblin TB, Sinkowitz-Cochran RL, Harris PL, Jacobs S, Liberatore K, Palfreyman MA, et al. Clinicians’ perceptions of the problem of antimicrobial resistance in health care facilities. Arch Intern Med. 2004;164(15):1662–8.
18 Skodvin B, Aase K, Charani E, Holmes A, Smith I. An antimicrobial stewardship program initiative: a qualitative study on prescribing practices among hospital doctors. Antimicrob Resist Infect Control. 2015;4:24.

19 James R, Luu S, Avent M, Marshall C, Thursky K, Buising K. A mixed methods study of the barriers and enablers in implementing antimicrobial stewardship programmes in Australian regional and rural hospitals. J Antimicrob Chemother. 2015;70(9):2665–70.
20 Pasay DK, Chow SJS, Bresee LC, Guirguis M, Slobodan J. Assessment of current antimicrobial stewardship policies and resources: a focus group project. Healthc Infect. 2015;20(1):7–15.
21 Bishop JL, Schulz TR, Kong DCM, Buising KL. Qualitative study of the factors impacting antimicrobial stewardship programme delivery in regional and remote hospitals. J Hosp Infect. 2019;101(4):440–6.
22 Pakyz AL, Moczygemba LR, VanderWielen LM, Edmond MB, Stevens MP, Kuzel AJ. Facilitators and barriers to implementing antimicrobial stewardship strategies: results from a qualitative study. Am J Infect Control. 2014;42 (10 Suppl):S257–63.
Competing interests: Other than the funding outlined above, no competing interests were declared. ( Return to Text )
Funding: This study was funded by a Development/Innovative Grant from the Nova Scotia Health Research Foundation. As a summer research student, Lindsay MacDonald received payment from the Nova Scotia Health Research Foundation and a Dalhousie University, Faculty of Health Professions establishment grant, to complete data collection. ( Return to Text )
The authors would like to acknowledge Steve Smith and Andrea Kent, of the Nova Scotia Health Authority, for their assistance in recruiting participants. The authors would also like to acknowledge Olga Kits from the Research Methods Unit at the Nova Scotia Health Authority for her support in reviewing the interview guide and advising on the analysis.
Canadian Journal of Hospital Pharmacy, VOLUME 72, NUMBER 4, July-August 2019