Lizanne Béïque, BPharm, PharmD, Luke Witherspoon, MSc, MB, MD, Rosemary Zvonar, BScPhm, ACPR, FCSHP, Kathryn N Suh, MD, MSc, FRCPC, Janet Squires, RN, PhD, Matthew Roberts, MD, FRCSC, Neal Rowe, MD, FRCSC, James Watterson, MD, FRCSC, Caroline Nott, MBBS, MSc, FRCPC
Pharmacists are essential members of antimicrobial stewardship programs, which have been in place for several years in many institutions, in response to the urgent threat posed by antibiotic resistance. It is well established that prolonged antibiotic exposure is associated with an increased risk of antimicrobial resistance, infection with Clostridioides difficile (previously known as Clostridium difficile), and adverse events1–3; however, research to optimize the duration of antibiotic therapy is still needed for many infections. During weekly antimicrobial stewardship rounds at the authors’ institution, it was noted that some patients presenting with an obstructive infected urinary stone were treated with a 2-week course of antibiotics, whereas others were treated with antibiotics until removal of the stone. Although guidelines recommend that removal of infected urinary stones not be undertaken until the infection has been adequately treated,4,5 the appropriate duration of antibiotic therapy has not been defined.4–8
To help address this gap in knowledge, we conducted a retrospective observational study to compare effectiveness and safety outcomes for patients admitted with sepsis secondary to one or more obstructive urinary stones, who were treated with the 2 most common durations of antibiotic therapy. The study was approved by the Ottawa Health Science Network Research Ethics Board. Patients 18 years of age or older who were admitted with an obstructive infected stone, who had undergone decompression (typically via urinary stenting), and who had been treated with either a 10- to 14-day course of antibiotics (± 2 days) followed by an antibiotic-free period until stone removal (group 1) or a longer, continuous course of antibiotics until stone removal (group 2) were included. Records of patients with the discharge diagnosis keywords (“stone”, “calculus”, or “calculi”) AND (“sepsis”, “septic”, “infected”, “urosepsis”, “UTI”, or “pyelonephritis”) from January 2014 to January 2017 inclusive were reviewed. The primary end point was recurrent infection (i.e., new antibiotic course or change in antibiotics prescribed for a urinary tract–related infection, on the basis of reported signs and symptoms, regardless of culture results) before stone removal. Secondary end points included recurrent infection between the time of stone and stent removal, stone- or stent-related complications, antibiotic-related adverse events and new microorganism resistance. The sample size needed was calculated as 49 patients per group, for a total of 98 patients. This sample size calculation was based on guidelines for chart audits.9 We based our calculation on a desired power of 0.8, precision of 0.2, α of 0.05, and expected proportion within the population with recurrent infection as 0.15. There were no previous studies to draw upon for determining the expected proportion; therefore, the estimate of 15% was conservative and was based on expert clinical opinion. Chi-square and Fisher exact tests were used for statistical analysis of the primary and secondary end points.
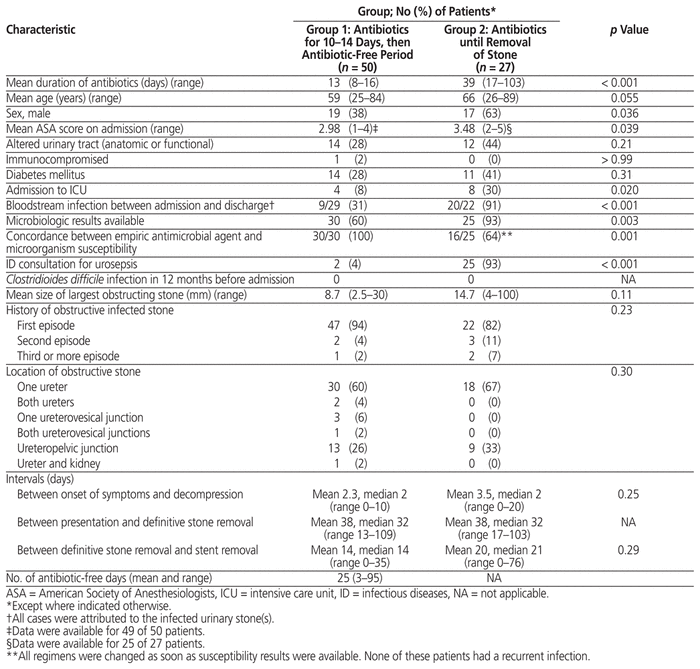
Because we had difficulty identifying patients for inclusion in group 2, we had fewer patients than planned: 50 patients in group 1 and 27 in group 2. Group 2 had significantly more men, higher American Society of Anesthesiologists scores, higher risk of not receiving an appropriate empiric antibiotic regimen, more blood-stream infections, more infectious diseases consultations, and more frequent admission to the intensive care unit relative to group 1 (Table 1). Primary and select secondary outcomes are presented in Table 2. All 8 patients with recurrent urinary tract infection before stone removal had received appropriate initial antibiotic therapy. In addition, among those for whom culture results were available (n = 5), the microorganism identified at the time of recurrent infection was different from that identified at the time of initial presentation, except for 1 patient, who was found to have a perinephric abscess. Infection with C. difficile occurred in 1 patient in group 1. New microorganism resistance was found in 2 urine specimens in each group. Antibiotic adverse events occurred in 1 patient in group 1 (diarrhea) and 2 patients in group 2 (rash, diarrhea).
Table 1 Patient Characteristics
Table 2 Recurrent Infections and Stone- or Stent-Related Complications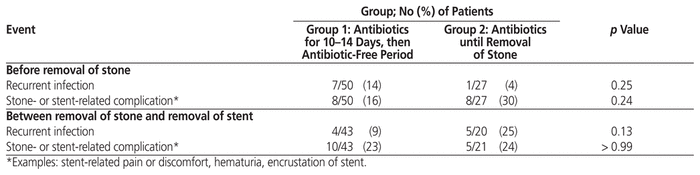
To the authors’ knowledge, this is the first published study to assess different durations of antibiotic therapy in patients with an obstructive infected urinary stone. In this study, patients in group 1 had a more than 3-fold increased risk of recurrent infection before stone removal relative to patients in group 2. This difference, while not statistically significant, may be clinically relevant.
Patients treated with a prolonged, uninterrupted course of antibiotics (group 2) were more likely to be male, were more likely to receive an ineffective empiric antibiotic, and were more severely ill on admission relative to the patients with an initial 10- to 14-day course of antibiotics followed by an antibiotic-free period (group 1). Despite these differences, patients in group 2 had a lower risk of recurrent infection before stone removal. If a difference between the 2 groups truly exists, these results suggest that a prolonged, uninterrupted course of antibiotics may be preferable. Alternatively, we hypothesize that the duration of the antibiotic-free period before definitive stone removal may have influenced the risk of recurrent infection, although this would need to be confirmed through further investigation. It is possible that a threshold of antibiotic-free days exists, beyond which the risk of recurrent infection increases. In both groups in our cohort, there was a wide range in the time to definitive stone treatment (Table 1), largely because of differences in access to operative time between surgeons.
Although there were no significant differences in the rate of new resistant microorganisms, C. difficile infections, and adverse drug events between groups 1 and 2, it is well established that the risk for these events increases with duration of antibiotic treatment.1–3 Given the lower number of patients we were able to enroll in group 2, our study may not have had sufficient power to detect any difference, even if such differences had been present. Other limitations include the retrospective nature of the study and the possibility that unassessed variables (e.g., antibiotics prescribed for non-urinary-tract-related infections after discharge, hydration status, potential missed events) may have contributed to the complications reported.
Although the optimal duration of treatment remains unresolved, these data may signal a difference in favour of a continuous course of antibiotics until definitive stone management, and they certainly provide an impetus to conduct a larger trial. Stewardship teams are well positioned to share these findings, while weighing the risks and potential benefits of both approaches.
1 Rice LB. The Maxwell Finland Lecture: For the duration — rational antibiotic administration in an era of antimicrobial resistance and Clostridium difficile. Clin Infect Dis. 2008;46(4):491–6.
2 Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011;53(1):42–8.
3 Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308–15.

4 Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP et al. Surgical management of stones: American Urological Association/Endourlogical Society guideline, part I. J Urol. 2016;196(4):1153–60.
5 Türk C, Neisius A, Petrik A, Seitz C, Skolarikos A, Thomas K. European Association of Urology guidelines on urolithiasis 2018. Arnhem (Netherlands): European Association of Urology; 2018 [cited 2018 Aug 28]. Available from: http://uroweb.org/guideline/urolithiasis/
6 Marien T, Miller NL. Treatment of the infected stone. Urol Clin North Am. 2015;42(4):459–72.
7 Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, et al. Medical management of kidney stones: AUA guideline. J Urol 2014;192(2):316–24.
8 Wollin DA, Joyce AD, Gupta M, Wong MYC, Lagunas P, Gravas S et al. Antibiotic use and the prevention and management of infectious complications in stone disease. World J Urol. 2017;35(9):1369–79.
9 Gregory B, Van Horn C, Kaprielian S. Eight steps to a chart audit for quality. Fam Pract Manag. 2008;15(7):A3–8.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors sincerely thank Amanda Owen for her assistance in collecting data and Pierre Giguère for his general guidance.
Canadian Journal of Hospital Pharmacy, VOLUME 72, NUMBER 4, July-August 2019