Najla Tabbara, Martha Brown, Allison McGeer, Andrew Wyllie
Influenza immunization is an effective strategy to reduce morbidity in health care providers and hospitalized patients.1 When the vaccine is not provided in prefilled glass syringes, pharmacies prepare doses in polypropylene syringes, from multidose vials, to facilitate administration during vaccination campaigns. The Canadian Immunization Guide recommends delaying the process of loading syringes until it is time to vaccinate the patient, because of the lack of data about vaccine stability in syringes.2 Data relating to room temperature storage are limited, and nonrefrigerated storage could result in reduced vaccine efficacy or adverse effects.2,3 However, refrigerators may not be consistently available during vaccination campaigns. Previous reports suggested that an influenza vaccine in its original prefilled glass syringe packaging can be stored for a period from 72 h to 14 days at room temperature without any effect on product quality.3,4 We sought to determine whether the influenza vaccine is stable in polypropylene syringes with refrigeration and at room temperature.
Vaccine stability testing includes determination of changes in vaccine structure, followed by immunologic assays to assess potency and biological activity.5 In the hemagglutination (HA) assay, the hemagglutinin protein protruding from the influenza vaccine envelope binds to red blood cells, causing them to agglutinate.6 This functional qualitative assay provides information about the physical stability of the vaccine.7 The current study used the HA assay to evaluate the stability of hemagglutinin, in terms of binding to its receptor, after storage of vaccine in polypropylene syringes.
Samples of the inactivated split-virion, trivalent influenza vaccine for the 2016/2017 season in the northern hemisphere (GlaxoSmithKline Inc, Mississauga, Ontario; lot 22TC5, expiry May 2017) were loaded into polypropylene syringes (Becton, Dickinson and Company, Franklin Lakes, New Jersey) and subjected to various storage conditions (all with protection from light). A 50-μL sample from each preparation was used to make serial 2-fold dilutions with phosphate-buffered saline in a 96-well round-bottom plate. Phosphate-buffered saline was used as the negative control, and freshly drawn-up vaccine was used as the positive control. Two drops of a 0.5% chicken red blood cell suspension were added to each well, and the plates were examined after 60 min at 4°C. A diffuse red layer at the bottom of the well was interpreted as indicating HA. In the absence of HA, the red blood cells settled as a “button”. Results were recorded by the study investigators, who were blinded to storage conditions. Results of HA activity are reported as geometric mean titres (GMTs), defined as the inverse of the highest dilution with complete HA. The GMT is a sensitive parameter used in immunohematological studies to detect differences in antibody effects.8,9 A GMT that is more than 2-fold lower than the positive control is interpreted as indicating a decrease in HA.7
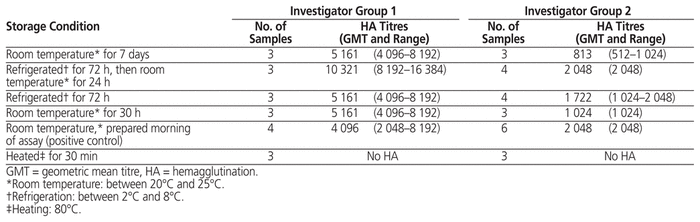
Two groups of investigators completed the experiment (Table 1). As expected, heated samples did not display any HA. For samples stored at room temperature for 7 days, the HA GMT for group 2 suggests that titres dropped during the storage period, although a 4-fold decrease in HA titre was observed in only 1 of 6 samples overall. The GMTs after storage under other conditions (refrigerated storage for 72 h, followed by room temperature storage for 24 h; refrigerated storage for 72 h; and room temperature storage for 30 h) were comparable to the GMTs of samples prepared the morning of the experiment and held at room temperature.
Table 1 Hemagglutination Activity of Inactivated Influenza Vaccine Loaded in Polypropylene Syringes and Stored under Various Conditions, as Tested by 2 Investigator Groups
The apparent decrease in HA titre after room temperature storage for 7 days may reflect recognized inter-rater variability in end-point detection of HA activity, or it may suggest that the structure of hemagglutinin is affected by extended exposure to room temperature. It would be of interest to test HA activity at intermediate times between 30 h and 7 days. However, storage in polypropylene syringes in select conditions (including refrigeration for 72 h and room temperature storage for 30 h) did not appear to reduce hemagglutinin activity, which indicates that storage in plastic did not lead to changes in protein structure. This finding suggests that potency would be retained, but quantitative serology assays, such as single radial immunodiffusion or viral neutralization assays, are required to confirm immunogenicity and clinical effect.5 Other limitations of our study include the use of a single brand of influenza vaccine from one season and a single brand of syringes. Considering the available evidence, storage of influenza vaccine samples in polypropylene syringes under refrigeration for 72 h and up to 30 h at room temperature maintains the ability of hemagglutinin to bind to its receptor, suggesting preservation of protein structure. These storage conditions could therefore facilitate vaccine preparation and administration during vaccination campaigns.
1 Cortes-Penfield N. Mandatory influenza vaccination for health care workers as the new standard of care: a matter of patient safety and nonmaleficent practice. Am J Public Health. 2014;104(11):2060–5.
2 Public Health Agency of Canada. Canadian immunization guide. Ottawa (ON): Government of Canada; [updated 2017; cited 2017 Dec 22]. Available from: https://www.canada.ca/en/public-health/services/canadian-immunization-guide.html
3 Cohen V, Jellinek SP, Teperikidis L, Berkovits E, Goldman WM. Room-temperature storage of medications labeled for refrigeration. Am J Health Syst Pharm. 2007;64(16):1711–5.
4 Coenen F, Tolboom JT, Frijlink HW. Stability of influenza sub-unit vaccine. Does a couple of days outside the refrigerator matter? Vaccine. 2006;24(4): 525–31.
5 WHO Expert Committee on Biological Standardization. Guidelines on stability evaluation of vaccines. Geneva (CH): World Health Organization; 2011 [cited 2019 June 5]. Available from: https://www.who.int/biologicals/vaccines/Annex_3_WHO_TRS_962-3.pdf?ua=1
6 Eisfeld AJ, Neumann G, Kawaoka Y. Influenza A virus isolation, culture and identification. Nat Protoc. 2014;9(11):2663–81.

7 Understanding influenza viruses: antigenic characterization. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases; 2017 [cited 2019 May 3]. Available from: https://www.cdc.gov/flu/about/viruses/index.htm
8 Guidelines on clinical evaluation of vaccines: regulatory expectations. Geneva (CH): World Health Organization; 2016 [cited 2019 May 3]. Available from: https://www.who.int/biologicals/expert_committee/Clinical_changes_IK_final.pdf
9 Leung VKY, Carolan LA, Worth LJ, Harper SA, Peck H, Tilmanis D, et al. Influenza vaccination responses: evaluating impact of repeat vaccination among health care workers. Vaccine. 2017;35(19):2558–68.
Competing interests and disclaimer: Grants for investigator-initiated research funding have been paid to Sinai Health System by GlaxoSmithKline and Sanofi Pasteur, for work by Alison McGeer unrelated to the study reported here. No company was involved in the design and conduct of the study; the collection, management, or interpretation of the data; or the preparation, review, or approval of the manuscript. No other competing interests were declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors thank Anthony Tham for his assistance with data collection.
Canadian Journal of Hospital Pharmacy, VOLUME 72, NUMBER 6, November-December 2019