Michelle Boyce, Anne Massicotte
Surgical site infections are an important cause of prolonged hospitalization, with an associated mortality rate of 3%.1 The incidence of a surgical site infection after surgery is 2% to 5%, and among surgical patients, such infections are the most common type of health care–associated infection.2 Patients who are receiving immunosuppressive therapy may be at increased risk of a postsurgical infection and delayed wound healing.3 In addition, multiple other contributing factors may increase the risk of infection after surgery, including (but not limited to) prolonged surgery (> 2 h), advanced age, obesity, smoking, cancer, other immunocompromising conditions, diabetes, and abdominal surgery.4 The risk of infection depends on whether the surgery is performed in a clean, sterile environment and considered low risk (e.g., cataract surgery, arthroscopy), or the surgery is performed in a contaminated, dirty environment and considered high risk (e.g., abdominal or gastrointestinal surgery).3,5 Furthermore, the type of surgical wound may be classified as clean, cleancontaminated, contaminated, and dirty/infected, as defined by the US Centers for Disease Control and Prevention,1 with each classification associated with a different degree of risk for a surgical site infection.4 At the same time, the severity of the patient’s underlying disease is an important factor to consider when determining perioperative drug management.6 For example, if the disease is severe, holding immunosuppressants may result in a negative outcome, such as a disease flare or relapse, whereas a patient with mild disease may tolerate temporary discontinuation of therapy. Hence, a risk–benefit assessment for each patient is warranted.6,7
This article aims to provide guidance to clinical practitioners for the perioperative management of rheumatology patients who are receiving immunosuppressive therapy and for whom elective surgery is planned. This guidance is a collection of recommendations from national rheumatology associations and other groups of rheumatology specialists. For the purpose of this review, immunosuppressive therapy includes common traditional disease-modifying antirheumatic drugs, as well as biologic agents used for rheumatoid diseases.
A formal literature search in Ovid MEDLINE and PubMed was conducted to gather relevant articles. The search terms, either as MESH words or keywords, were disease modifying antirheumatic drug*, DMARD*, immunosuppressive agents, biologic*, monoclonal antibodies, tumor necrosis factor-alpha, rheumatic diseases, practice guideline*, recommendation*, consensus, surgical procedures, surgery, and operative (peri, pre, intra, post). Searches were limited to guidelines and review articles addressing the perioperative use of immunosuppressants. A general Google search was also performed to capture other possibly relevant material that would not have been formally indexed. After removal of duplicates, irrelevant articles (i.e., those that did not substantially address our topic), and articles written in languages other than French or English, 4 national guidelines and 4 review articles remained as the best available evidence. Most of the data that we reviewed focused on patients with rheumatic diseases, and we therefore limited this guidance document to this patient population.
The literature search revealed a lack of prospective studies establishing the optimal withhold and restart times for immunoCJHP suppressants during the perioperative period. As such, the recommendations and reviews retrieved through the literature search focused on guiding principles (e.g., type of surgery, drug half-life, and drug dosing interval).
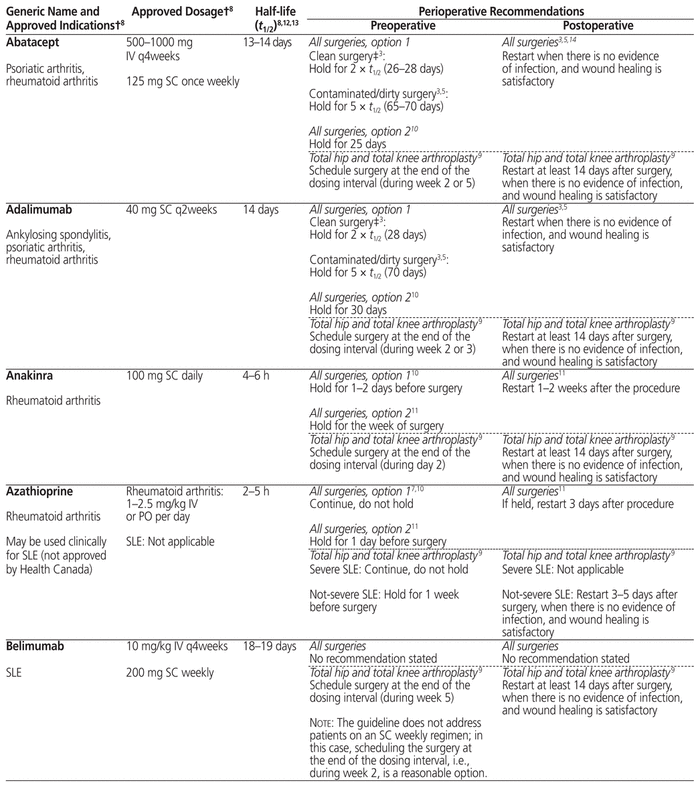
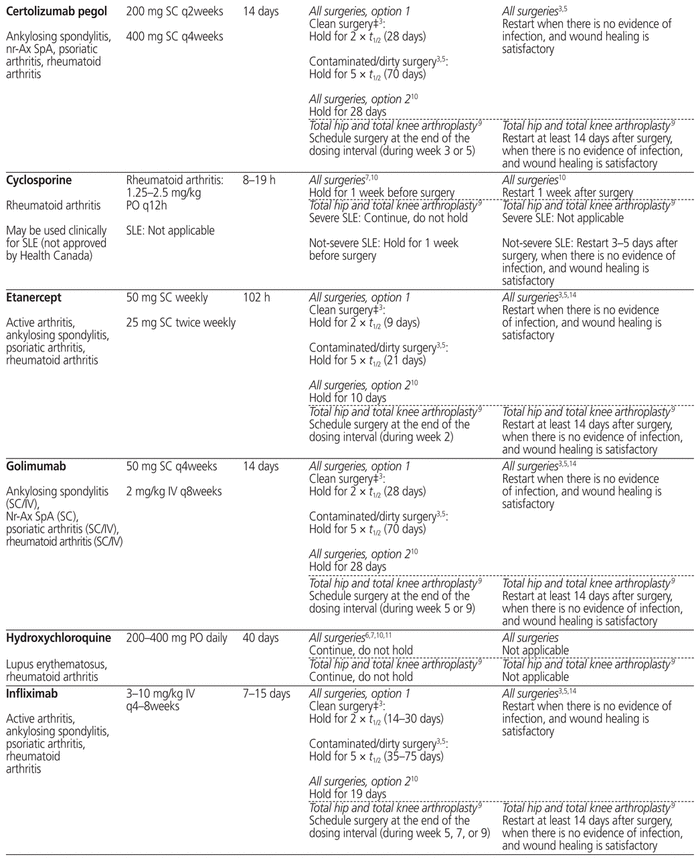
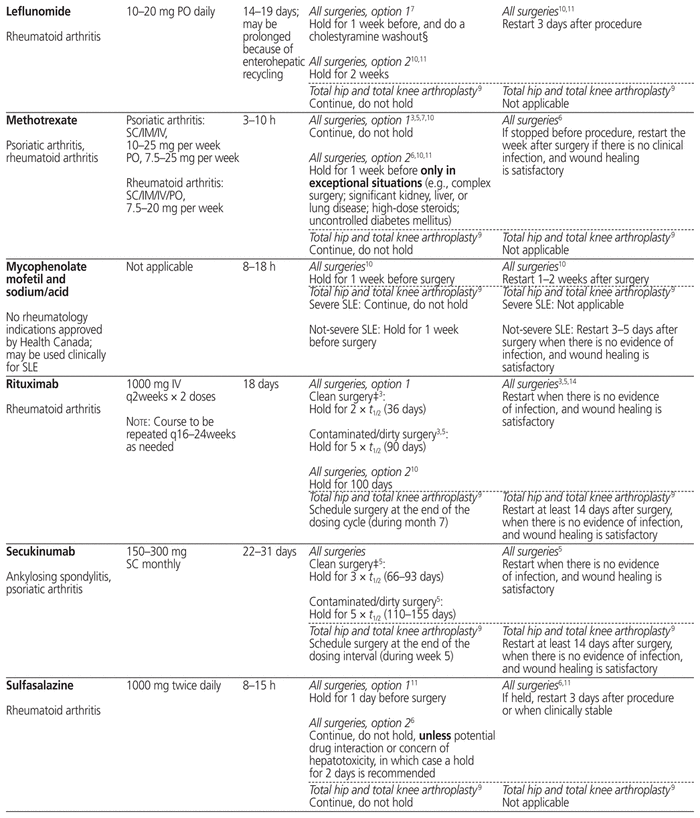
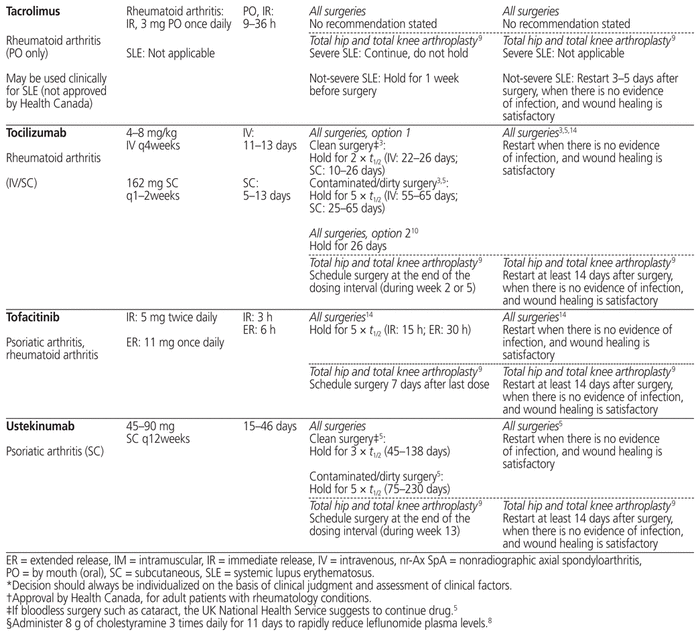
Table 1 summarizes management recommendations for rheumatology patients during the perioperative period of elective surgeries for common immunosuppressants marketed in Canada.3,5–14 Canadian recommendations have been prioritized as much as possible (in Table 1, see the recommendations originating from reference 3). The information in this table applies only to rheumatology patients and does not cover other populations, such as transplant patients, who may be at risk of organ rejection if immunosuppressive therapy is stopped temporarily.
Table 1 Perioperative Management of Immunosuppressive Therapy for Adult Rheumatology Patients*



The table separates “all surgeries” from “total hip and total knee arthroplasty” for the following 2 reasons: first, the references cited in these 2 categories adopted a very different approach for the perioperative management of immunosuppressants, and second, the US recommendations are specific to patients undergoing elective total hip or total knee arthroplasty. The table also provides, in many cases, 2 different options for “all surgeries” (i.e., not limited to a specific type of surgery), reflecting the lack of consensus on the perioperative management of immunosuppressants.
When determining the period for which a drug should be held before surgery, the elimination half-life (t1/2) of each immunosuppressant and its metabolites is a useful tool.3,5,14 Most of the guidelines recommend holding a drug for 2 to 3 half-lives if the surgery carries a low risk of infection, and for 5 half-lives if the surgery carries a high risk of infection.3,5 In Table 1, the minimum of 2 (or in some cases 3) half-lives and maximum of 5 half-lives are stated with the actual calculated time in parentheses for each drug; if there is a range of half-lives, the range of time to hold the drug is stated. The reported half-life of a particular drug may differ among sources in the literature, and therefore the time to hold the drug, as stated in Table 1, may differ slightly from the quoted references. Clinical judgment will be of primary importance when applying these recommendations to special populations such as elderly patients and those with renal or hepatic impairment, given likely differences in pharmacokinetic parameters.
In the context of total hip and total knee arthroplasty, the recommendations in the US guidelines are based on the drug dosing interval rather than drug half-life, because the half-life does not always correlate with each drug’s duration of action.9 The US recommendation is to schedule the surgery at the end the drug dosing interval, when it would normally be the time to proceed with the next dose.9
In addition to the type of surgery, drug half-life, and drug dosing interval, addressed in Table 1, the final decision about the exact duration of drug-holding should still be individualized according to patient-specific risk factors and comorbidities.
Before restarting an immunosuppressant postoperatively, evaluation of the wound is important to ensure adequate healing, because re-initiation of immunosuppressive therapy too early can put the patient at increased risk of postoperative infection. Most of the available guidelines recommend resuming the immunosuppressive therapy when there are no signs of infection and there is evidence of satisfactory wound healing.3,5,14
A 34-year-old woman with breast cancer is scheduled to undergo an elective mastectomy. She has rheumatoid arthritis that has been well controlled over the past 2 years with adalimumab 40 mg SC every 2 weeks and methotrexate 7.5 mg orally once weekly. She has no renal or hepatic impairment. Using Table 1 as a guide, we could recommend holding the adalimumab for 28 days before surgery (given that a mastectomy is generally classified as a clean surgical procedure) and continuing the methotrexate throughout the perioperative period. The patient could resume adalimumab therapy when there is no evidence of infection and wound healing is satisfactory.
A 60-year-old man with psoriatic arthritis receives infliximab by infusion every 4 weeks, with his most recent infusion administered on March 2. The patient has responded well to infliximab and has not experienced any flares of his disease in the past year. He is scheduled to undergo an elective total hip arthroplasty. The orthopedic surgeon is wondering for how long the infliximab should be held before the surgery. According to Table 1, it would be best to schedule the surgery during the week of March 30 (at the end of the infliximab dosing interval, i.e., during week 5) and to hold the dose scheduled for March 30. The patient could resume his infliximab infusions at least 14 days after surgery, when there is no evidence of infection and wound healing is satisfactory.
Patients who are receiving immunosuppressive therapy may be at increased risk of infection after surgery; therefore, holding immunosuppressants may be warranted in the perioperative period. However, holding immunosuppressants may result in a flare of the underlying disease. This review has summarized practical guidance addressing this issue for rheumatology patients. Table 1 is provided as a guide in the decision-making process, but final decisions should be tailored to each patient, balancing the risks and benefits of holding or continuing therapy. Factors to consider when deciding to continue or hold an immunosuppressant drug include the type of surgery, comorbidities, severity of the disease, and any other factor that could contribute to the patient’s risk of infection.3 If it is decided to hold the drug before surgery, a general guide of holding the drug for 2 to 5 half-lives may be used, unless the planned surgery is an elective total hip or total knee arthroplasty, for which use of the dosing-interval method is suggested. There is a general consensus that immunosuppressive therapy should be resumed when there is no evidence of infection and wound healing is satisfactory. Because clinical data and guidelines are few, there is a need for further research to develop a standardized approach for optimizing perioperative care of these patients.6,7
1 Module 9: Surgical site infection (SSI) event [procedure-associated module]. Centers for Disease Control and Prevention; 2020 [cited 2020 May 26]. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
2 Canadian surgical site infection prevention audit month February 2016: recap report. Canadian Patient Safety Institute; 2016 [cited 2019 May 5]. Available from: www.patientsafetyinstitute.ca/en/toolsResources/Documents/SSIAudit2016_Recap Report EN.pdf
3 Bombardier C, Hazelwood GS, Akhavan P, Schieir O, Dooley A, Haraoui B, et al. Canadian Rheumatology Association recommendations for the pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs: part II safety. J Rheumatol. 2012;39(8):1583–602.
4 Causes and risk factors of surgical site infections. In: Surgical site infections. Johns Hopkins University; [cited 2019 May 5]. Available from: https://www.hopkinsmedicine.org/healthlibrary/conditions/adult/dermatology/surgical_site_infections_134,144
5 Kerrigan N. Guidelines for the management of interruption of biologic therapies for elective surgery in adults and children with rheumatoid arthritis, JIA and ankylosing spondylitis. NHS Foundation Trust (UK): 2017.
6 Härle P, Straub RH, Fleck M. Perioperative management of immuno-suppression in rheumatic diseases—what to do? Rheumatol Int. 2010; 30(8):999–1004.
7 Koons K, Plotas V, Tichansky DS, Kammerer MR. The safety of elective surgery with concurrent use of immunosuppressants. Glob Surg. 2017;3(2): 1–4.
8 Heath Canada drug product database [database on internet]. Health Canada; [cited 2019 May 5]. Available from: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp
9 Goodman SM, Springer B, Guyatt G, Abdel MP, Dasa V, George M, et al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons guideline for the perioperative management of antirheumatic medication in patients with rheumatic diseases undergoing elective total hip or total knee arthroplasty. Arthritis Care Res. 2017;69(8):1111–24.
10 Franco AS, Iuamoto LR, Pereira RM. Perioperative management of drugs commonly used in patients with rheumatic diseases: a review. Clinics. 2017;72(6):386–90.

11 Rosandich PA, Kelley JT, Conn DL. Perioperative management of patients with rheumatoid arthritis in the era of biologic response modifiers. Curr Opin Rheumatol. 2004;16(3):192–8.
12 Lexi-Drugs online [clinical database]. In: Lexicomp online. Wolters Kluwer; [cited 2019 May 5]. Accessed through institutional portal; subscription required to access content.
13 IBM Micromedex [database on internet]. IBM; [cited 2019 May 5]. Accessed through institutional portal; subscription required to access content.
14 Louthrenoo W, Kasitanon N, Katchamart W, Aiewruengsurat D, Chevaisrakul P, Chiowchanwisawakit P, et al. 2016 updated Thai Rheumatism Association recommendations for the use of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20(9):1166–84.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors would like to acknowledge Alexandra (Sascha) Davis, Librarian with The Ottawa Hospital, for her guidance with the literature search; and Yasmin Khaliq, who was at the time of the original submission a Pharmacist with the Ottawa Valley Regional Drug Information Centre and The Ottawa Hospital, for her presubmission editing assistance.
Canadian Journal of Hospital Pharmacy, VOLUME 73, NUMBER 3, May-June 2020