Stability of Extemporaneously Compounded Suspensions of Trimethoprim and Sulfamethoxazole in Amber Plastic Bottles and Amber Plastic Syringes
Isabelle
St-Jean
,
M Mihaela
Friciu
,
Anaëlle
Monfort
,
Jessica
MacMahon
,
Jean-Marc
Forest
,
Scott
Walker
,
Grégoire
Leclair
ABSTRACT
Background
Trimethoprim (TMP) and sulfamethoxazole (SMX) are widely used, in combination, to treat or prevent various infections. Unfortunately, no liquid oral formulation is currently available in Canada for patients who are unable to swallow tablets.
Objective
To evaluate the stability of suspensions of TMP and SMX (8 and 40 mg/mL, respectively) prepared in Oral Mix or Oral Mix SF vehicle (Medisca Pharmaceutique Inc) and stored for up to 90 days in amber plastic bottles or amber plastic syringes at 5°C or 25°C.
Methods
Suspensions were prepared from bulk powder and from tablets in Oral Mix and Oral Mix SF vehicles, then transferred to amber plastic (polyethylene terephthalate glycol) bottles and plastic oral syringes and stored at 5°C and 25°C. Samples were collected on predetermined study days (0, 7, 14, 23, 45, 60, 75, and 90 days) and analyzed using a validated high-performance liquid chromatography – ultraviolet detection method. A suspension was considered stable if it maintained at least 90% of its initial concentration with 95% confidence. Observations of organoleptic characteristics such as colour and odour, as well as pH, were used to assess physical stability.
Results
Suspensions prepared from bulk powder maintained concentrations of TMP and SMX of at least 97% of the initial concentration over the 90-day study period. No obvious changes in colour, odour, or pH were observed. However, acceptable suspensions could not be prepared from the commercial tablets. A persistent foam that developed at the surface of all suspensions prepared from tablets could result in inconsistent dosing.
Conclusions
Extemporaneously compounded oral suspensions of TMP and SMX (8 and 40 mg/mL, respectively) prepared from bulk powder in Oral Mix and Oral Mix SF vehicles and stored in amber plastic bottles or syringes at 5°C or 25°C remained stable for at least 90 days. Suspensions made from tablets produced unacceptable formulations.
KEYWORDS:
trimethoprim
,
sulfamethoxazole
,
stability
,
compounded oral suspension
,
Oral Mix
,
Oral Mix SF
RÉSUMÉ
Contexte
Le triméthoprime (TMP) et le sulfaméthoxazole (SMX) sont largement utilisés conjointement pour traiter ou prévenir diverses infections. Malheureusement, aucune formulation liquide orale n’est actuellement disponible au Canada pour les patients incapables d’avaler des comprimés.
Objectif
Évaluer la stabilité des suspensions de TMP et de SMX (respectivement 8 et 40 mg/mL) préparées dans un véhicule Oral Mix ou Oral Mix SF (Medisca Pharmaceutique Inc.) et stockées pendant 90 jours dans des flacons ou des seringues en plastique ambré à 5 °C ou 25 °C.
Méthodes
Les suspensions ont été préparées à partir de poudre en vrac et de comprimés dans les véhicules Oral Mix et Oral Mix SF, puis transférées dans des flacons en plastique ambré (polyéthylène téréphtalate glycol) et dans des seringues orales en plastique et stockées à 5 °C et 25 °C. Des échantillons ont été recueillis à des jours prédéterminés (0, 7, 14, 23, 45, 60, 75 et 90 jours) et analysés à l’aide d’une méthode de détection par ultraviolet validée de chromatographie en phase liquide à haute performance. La suspension était jugée stable si elle préservait au moins 90 % de sa concentration initiale avec un seuil de confiance de 95 %. Les observations des caractéristiques organoleptiques, comme la couleur et l’odeur, ainsi que le pH, ont été faites pour évaluer la stabilité physique.
Résultats
Les suspensions préparées à partir de poudre en vrac préservaient au moins 97 % de la concentration initiale de TMP et de SMX pendant la période d’étude de 90 jours. Aucun changement manifeste de couleur, d’odeur ou de pH n’a été observé. Cependant, les suspensions acceptables n’ont pas pu être préparées à partir des comprimés commerciaux. Une mousse homogène se formait à la surface de ces suspensions, ce qui pourrait entraîner un dosage incohérent.
Conclusions
Les suspensions orales composées extemporanées de TMP et SMX (respectivement 8 et 40 mg/mL) préparées à partir de poudre en vrac dans des véhicules Oral Mix et Oral Mix SF et stockées dans des flacons ou des seringues en plastique ambré à 5 °C ou 25°C sont restées stables pendant au moins 90 jours. Les suspensions préparées à partir de comprimés ont donné des formulations inacceptables.
MOTS CLÉS:
triméthoprime
,
sulfaméthoxazole
,
stabilité
,
suspension orale composée
,
Oral Mix
,
Oral Mix SF
INTRODUCTION
Trimethoprim-sulfamethoxazole (TMP-SMX) is often used to treat various infections, including infections of the urinary tract, respiratory tract, and gastrointestinal system.1 TMP-SMX is widely used as routine prophylaxis for
Pneumocystis jirovecii
(formerly known as
Pneumocystis carinii
) pneumonia in immunocompromised patients, including pediatric oncology patients, as well as patients with congenital and acquired severe immune deficiency.2 Before the use of TMP-SMX prophylaxis,
P. jirovecii
pneumonia, a life-threatening infection, occurred in up to 43% of children with cancer.3 TMP-SMX is the drug of choice for prophylaxis against this disease because of its high efficacy, as well as its tolerability, low cost, and broad antimicrobial spectrum.4
Oral suspensions of TMP-SMX are useful for patients who are unable to swallow tablets, which can occur because of conditions such as dysphagia and mucositis or simply young age. Unfortunately, the commercially available oral suspensions from Apotex5 and Teva6 have been in short supply in Canada since 2017, a situation expected to continue for an unknown period. To date, only one study concerning the stability of compounded TMP-SMX suspension has been published.7 In that study, suspensions of TMP and SMX (8 and 40 mg/mL, respectively) in simple syrup were stable for only 20 days with storage at 4°C in amber plastic bottles. However, simple syrup has some disadvantages, particularly for pediatric formulations, because it does not properly mask the taste of the active ingredient and it does not contain an appropriate suspending agent for longer-term storage. Also, suspensions made from Apo-sulfatrim and Teva-trimel tablets in simple syrup, as described in the previous study,7 were thick, with cakes formed at the surface, which led to difficulty in homogenizing the suspensions. Moreover, no data exist concerning the stability of suspensions of TMP-SMX (8 and 40 mg/mL, respectively) in dye-free Oral Mix and Oral Mix SF (sugar-free) vehicles (Medisca Pharmaceutique Inc).8 These vehicles contain flavouring and preservatives that mask the taste of the medications and permit long-term stability.
The objective of this study was to determine the physical and chemical stability of oral suspensions of TMP and SMX (8 and 40 mg/mL, respectively) in Oral Mix and Oral Mix SF vehicles when stored at 5°C or 25°C in amber plastic bottles or oral plastic syringes for up to 90 days.
METHODS
Compounded Preparations from Bulk Powder and Tablets
Suspensions of TMP and SMX (8 and 40 mg/mL, respectively) were prepared from bulk powder and from tablets. For the bulk powder preparations, TMP USP micronized 98.6% powder (Medisca Pharmaceutique Inc, lot 611580/K) and SMX EP 99.9% powder (Medisca Pharmaceutique Inc, lot 610450/D) were first accurately weighed and then mixed together in a mortar using a pestle, before geometric incorporation of either Oral Mix (Medisca Pharmaceutique Inc, lot 611853/B) or Oral Mix SF (Medisca Pharmaceutique Inc, lot 611850/A) vehicle for a final volume of 150 mL. This operation was repeated 3 times to prepare 3 independent batches of suspension. Suspensions of the same total volume (150 mL) were similarly prepared from 15 pulverized tablets containing TMP and SMX (80 and 400 mg, respectively; Apo-sulfatrim, Apotex Inc, lot MX5200).
Design of Stability Study
Each 150-mL suspension was subdivided and packaged in 50-mL amber plastic (polyethylene terephthalate glycol) bottles (2 bottles of 50-mL fill volume per formulation per batch; Medisca Pharmaceutique Inc, lot 600990/A) and 3-mL amber plastic oral syringes (16 syringes of 2.5-mL fill volume per formulation per batch; PreciseDose Dispenser, Medisca Pharmaceutique Inc, lot 617025/B) and stored at 5°C or 25°C for up to 90 days. The remaining quantity of each suspension (10 mL) was discarded. For each formulation, 3 bottles (one per batch) were stored at each temperature; similarly, 3 syringes (one per batch) for each time point were stored at each temperature.
At each time point (0, 7, 14, 23, 45, 60, 75, and 90 days), a 2.5-mL aliquot from each bottle and 3 syringes per preparation were retrieved from each temperature condition. The bottles and syringes were vigorously shaken and vortex-mixed before sampling. For each test sample, odour and colour were inspected, the pH was measured, and samples were collected for later determination of the concentrations of TMP and SMX by high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection (for practical reasons, the samples collected at each time point were frozen at −80°C, and all samples were analyzed on the same day). This design ensured that all stability conditions consisted of 3 separately prepared and stored formulations (experimental
n
= 3). Furthermore, each sample was analyzed twice by HPLC (technical
n
= 2).
Physical Properties
The physical properties of the suspensions prepared from bulk powder and from tablets were evaluated over the 90-day study period. At each time point, samples were examined for obvious changes in appearance and odour, and the pH was measured (model AP61 pH meter, Fisher Scientific). A difference in pH relative to initial measured value of not more than 1 unit was considered acceptable. The pH meter was calibrated at the beginning of each study day using commercially available standards.
Liquid Chromatography
HPLC-UV Method
The HPLC system (model Prominence UFLC, Shimadzu) was equipped with an LC-20AD binary pump operating at a flow rate of 1 mL/min, a DGU-20A5 solvent degasser, an SPD-M20A multiple-wavelength photodiode array detector set at 240 nm for TMP and 270 nm for SMX, an SIL-20AC HT refrigerated autosampler at 5°C, and a CTO-20AC column oven at 25°C. A Zorbax RX-C18 column (4.6 × 150 mm, 5 μm, Agilent Technologies Canada) was used for the study. Mobile phases consisted of 20 mmol/L aqueous monobasic potassium phosphate (JT Baker Inc, lot Y22465) adjusted to pH 2.5 using phosphoric acid and methanol (83:17; Fisher Scientific, lot 144689). The drugs were quantified using the area of the peak eluting at approximately 7.6 minutes for TMP and 11.2 minutes for SMX.
Assay Validation
The assay was validated by evaluating the accuracy and reproducibility of the standard curves on 3 different days. On each validation day, suspensions of TMP 9.6 mg/mL and SMX 48 mg/mL were prepared from Medisca bulk powders in Oral Mix and Oral Mix SF vehicles. Medisca powders were chosen over reference standards to produce the calibration standards because of their lower cost. The stock solutions were then diluted with each vehicle to obtain solutions of 6.4, 7.2, 8.0, 8.8, and 9.6 mg/mL for TMP and 32, 36, 40, 44, and 48 mg/mL for SMX. Samples (100 μL) of these solutions were diluted with methanol (10 mL) in 15-mL centrifuge tubes. Each mixture was vortex-mixed (20 seconds) and then centrifuged (3400 rpm, 15 minutes). Supernatant (300 μL) was diluted with water (600 μL) to yield standards of 21.33, 24.00, 26.66, 29.33, and 32.00 μg/mL for TMP and 106.67, 120.00, 133.33, 146.67, and 160.00 μg/mL for SMX. These standards were analyzed by HPLC in triplicate to create the standard curve.
Intraday variability was evaluated by injecting standard samples in triplicate within the same day, and interday variability was evaluated by injecting standard samples on 3 different days. Finally, intraday and interday errors were assessed from the coefficients of variation of the peak areas of each standard.
Standard Curve and Sample Preparation for HPLC Injection
For the HPLC analysis on the assay day, fresh standard curves and test samples were prepared and diluted as described in the assay validation section. These solutions for injection, with nominal concentrations of 26.67 μg/mL for TMP and 133.33 μg/mL for SMX, were analyzed in duplicate by HPLC immediately after preparation.
Forced Degradation of TMP and SMX
Suspensions of TMP and SMX (8 and 40 mg/mL, respectively) were prepared from bulk powder in Oral Mix and Oral Mix SF vehicles, as described above. Forced degradation was performed by mixing 0.5 mL of each suspension with either 0.5 mL of water, 0.5 mL of aqueous hydrochloric acid 1 mol/L, 0.5 mL of aqueous sodium hydroxide 1 mol/L, or 0.5 mL of aqueous hydrogen peroxide 30%. The solutions were stored for 3 hours at 60°C, except for a second solution in water stored for 3 hours at 4°C, then treated as previously described and analyzed by HPLC. The chromatograms obtained from these degradation analyses were compared with chromatograms obtained from TMP-SMX in Oral Mix and Oral Mix SF (10 mg/mL) diluted 1:1 with water and analyzed by HPLC to look for any changes in concentration, retention time, and peak shape. Finally, the chromatograms were inspected for additional peaks.
Statistical Analysis
For each combination of suspension type, container, and storage temperature, the mean was calculated for the 3 samples, each assayed in duplicate. The percent remaining was analyzed by linear regression, and a 95% confidence interval (CI) was constructed around the slope of percent remaining versus study days. The time to achieve 90% of the initial concentration (T-90) with 95% confidence (expressed as “T-90
95%CI
”) was calculated from the time (in days) for the lower limit of the 95% CI to reach 90%. Analysis of variance and multiple linear regression were used to test differences in concentration on different study days, with different suspending agents, containers, and temperatures for both TMP and SMX. The 5% level was used as the a priori cut-off for significance.
Concentrations of TMP and SMX were considered “acceptable” or “within acceptable limits” if the lower limit of the 95% CI of concentration remaining (T-90
95%CI
) was greater than 90% of the initial (day 0) concentration.
RESULTS
Physical Study
The suspensions prepared from bulk powder had a uniform appearance and good “pourability” and were easily redispersed after settling. No notable changes in colour (white) or odour (sweet cherry) were observed after storage under different conditions for 90 days. Moreover, the difference in pH relative to initial pH was not more than 0.2 unit for these preparations under all tested conditions. Taste was not evaluated during this study.
However, the suspensions prepared from tablets were not acceptable. Indeed, the suspension prepared with Oral Mix SF vehicle was highly viscous, and this formulation was therefore not included in the study. The suspension prepared with Oral Mix vehicle was less thick, but a persistent layer of foam developed at the surface of the suspension, which made redispersion difficult after settling and resulted in inconsistent sampling and high variability in measured concentrations. Apo-sulfatrim tablets include methylcellulose, a surfactant that might have caused the foam. Other commercial tablets, such as Teva-trimel tablets, contain sodium lauryl sulfate, a surfactant that could also lead to foaming.1 However, the latter tablets were not tested in our study.
Overall, the results obtained with suspensions prepared from tablets were not consistent and are not reported.
Assay Validation
Regression analysis of the peak area of TMP and SMX versus the concentration of each TMP and SMX standard demonstrated linearity over the range of concentrations tested, with coefficients of determination (
r
2) of at least 0.99993 for TMP and 0.9998 for SMX. As described above, all test samples were first frozen at −80°C during the study and then analyzed on the same assay day. On the assay day, the coefficients of determination were at least 0.99996 for TMP and 0.9998 for SMX.
During validation, the highest intraday coefficients of variation for the standards, calculated for triplicate injection samples, were 0.46% for TMP and 0.35% for SMX, and the highest interday coefficients of variation over 3 days were 3% for TMP and 2% for SMX. On the assay day, the highest intraday coefficients of variation for triplicate injection samples of the standards were 0.39% for TMP and 0.35% for SMX. Moreover, a combined standard of TMP 26.66 μg/mL and SMX 133.33 μg/mL was analyzed every 24 injections. For this standard, the highest intraday coefficient of variation, calculated for 7 injections, was 0.20% for TMP and 0.35% for SMX.
Forced Degradation
No peak overlap of TMP and SMX with excipients, impurities, or degradation products was observed during forced degradation. Similarity of the UV spectra from all sampling points on the peak was compared using the HPLC system software (LabSolution v. 5.54, Shimadzu) to compute a similarity index and determine the presence of multiple components within the peak. The similarity index ranges between −1 (dissimilar) and +1 (identical).9 The peak purity index calculated between 250 and 310 nm was not less than 0.9999 in all cases.
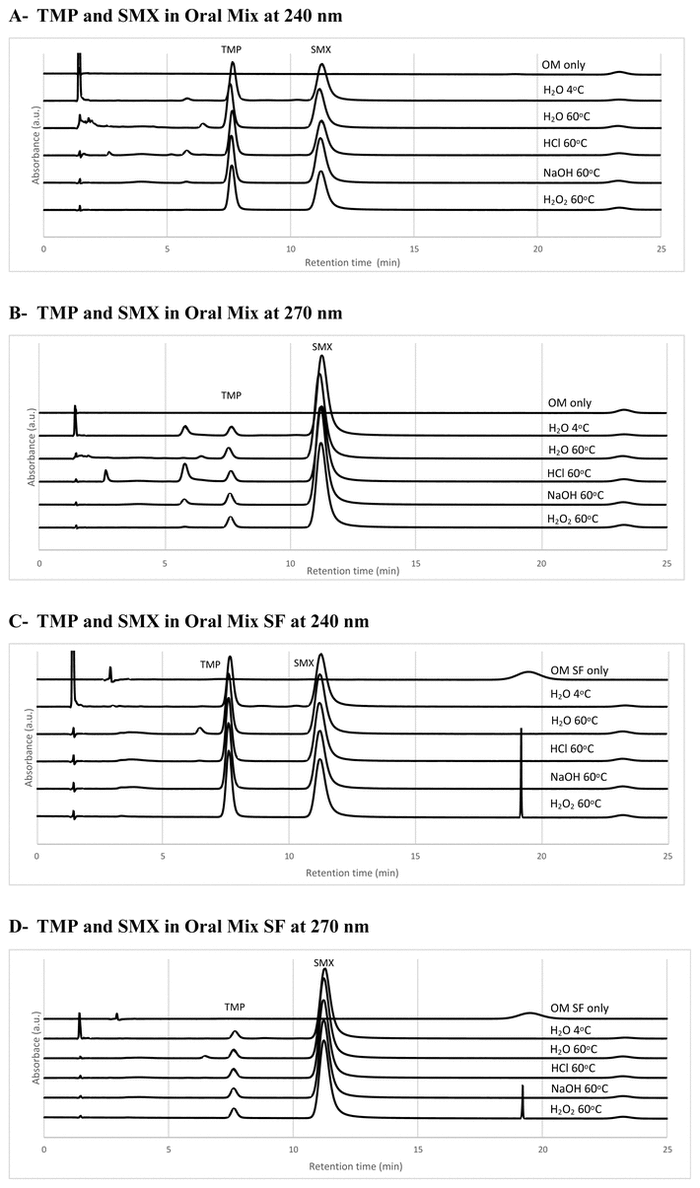
In Oral Mix vehicle, the peak for TMP was not reduced in water, HCl, or NaOH and was reduced by 14% in H
2
O
2
; in Oral Mix SF vehicle, the peak for TMP was reduced by 2% in water, 5% in HCl, 10% in NaOH, and 22% in H
2
O
2
(Figure 1). In Oral Mix vehicle, the peak for SMX was not reduced in water or NaOH and was reduced by 11% in HCl and 6% in H
2
O
2
; in Oral Mix SF vehicle, the peak for SMX was not reduced in water, HCl, or NaOH and was reduced by 9% in H
2
O
2
(Figure 1). Furthermore, no interference from vehicles was observed, as shown in the chromatogram with water at 4°C in Figure 1. The HPLC method was therefore considered stability-indicating.
| |
|
|
|
FIGURE 1
Panels A and B: Representative chromatograms of Oral Mix (OM) only, as well as trimethoprim (TMP; retention time 7.6 minutes) and sulfamethoxazole (SMX; retention time 11.3 minutes) in Oral Mix, analyzed at 240 and 270 nm (for TMP and SMX quantification, respectively), in water at 4°C and after forced degradation in water, hydrochloric acid 1 mol/L, sodium hydroxide 1 mol/L, and hydrogen peroxide 30% at 60°C. Panels C and D: Representative chromatograms of Oral Mix SF (OM SF) only and TMP and SMX in Oral Mix SF at 240 and 270 nm in water at 4°C and after forced degradation in water, HCl 1 mol/L, NaOH 1 mol/L, and H
2
O
2
30% at 60°C.
|
Chemical Stability and Statistical Analysis
The concentrations of TMP and SMX in Oral Mix or Oral Mix SF vehicle, prepared from bulk powder, were not less than 97% of the initial concentration after storage in amber plastic bottles or amber plastic syringes at 5°C or 25°C for up to 90 days (Tables 1 and 2).
TABLE 1
Proportion of Initial Concentration of Trimethoprim Remaining (as Mean % ± Relative Standard Deviation) on Each Study Day, after Preparation from Bulk Powder in Oral Mix and Oral Mix SF Vehicles and Storage in Amber Plastic Bottles and Amber Plastic Syringes at 5°C and 25°C
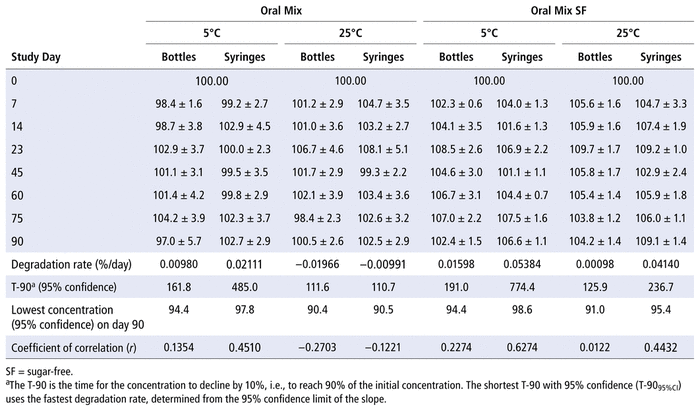
TABLE 2
Proportion of Initial Concentration of Sulfamethoxazole Remaining (as Mean % ± Relative Standard Deviation) on Each Study Day, after Preparation from Bulk Powder in Oral Mix and Oral Mix SF Vehicles and Storage in Amber Plastic Bottles and Amber Plastic Syringes at 5°C and 25°C
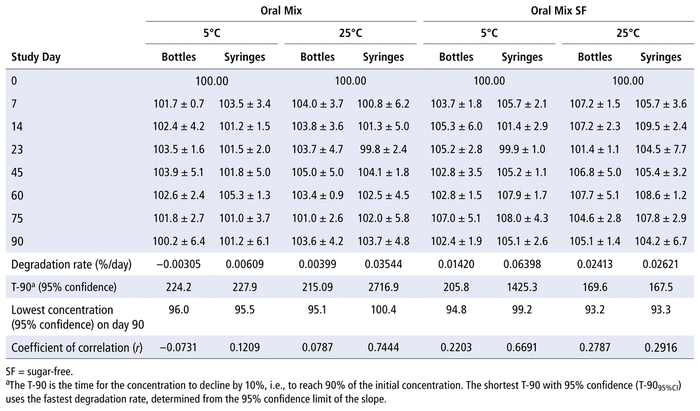
The 95% confidence limits constructed around the concentrations on the last study day exceeded 90% for TMP and 93% for SMX for all combinations of container, suspension vehicle, and storage temperature. Analysis of variance detected differences in the percent remaining for both TMP and SMX due to study day (
p
< 0.001), suspending agent (
p
< 0.001), temperature (
p
< 0.001 for TMP,
p
= 0.031 for SMX), and container (
p
= 0.037 for TMP,
p
= 0.90 for SMX). Multiple linear regression also detected differences in the percent remaining due to suspending agent (
p
< 0.001) and temperature (
p
= 0.004 for TMP,
p
< 0.001 for SMX). There was no significant relation with container (
p
= 0.10 for TMP,
p
= 0.92 for SMX). The study method was able to detect differences in concentration of 2% or more for both TMP and SMX.
Analysis of the concentration results support a before-use date (BUD) of 90 days at both temperatures and with all combinations of container and suspending agent.
DISCUSSION
The results of the HPLC analysis showed that suspensions of TMP and SMX (8 and 40 mg/mL, respectively) prepared from bulk powder in Oral Mix and Oral Mix SF maintained at least 97% of their original concentrations for the entire 90-day study period with storage at 5°C or 25°C in amber plastic bottles or amber plastic syringes. Analysis of the concentration results supports a BUD of 90 days at both temperatures and with all combinations of container and suspending agent. This indicates that there is less than a 2.5% chance that after 90 days of storage the concentration of either TMP or SMX will be less than 90% of the initial concentration.
Inspection of Table 1 reveals some positive degradation rates. Positive (as well as negative) degradation rates have been reported from previous studies in which the study drug degraded very slowly. This is the result of an interaction between analytical variability and slow degradation rate, such that random error results in the appearance of a positive degradation rate. It is for this reason that confidence intervals are required for analyzing stability data, because they combine the average degradation rate and the analytical variability, providing BUDs that are useful to pharmacists (i.e., there is less than a 2.5% chance that concentrations identified in Table 1 as the lowest concentrations would ever be observed in clinical practice on the 90th day of storage).
No previous study has demonstrated the physical and chemical stability of extemporaneously prepared TMP-SMX suspensions for this period of time under these conditions. These results are important as they will allow pharmacists to compound this suspension with a BUD longer than 20 days. The only previously published study of this drug combination7 evaluated the stability of TMP and SMX in suspensions stored for 20 days, with the compounded formulations prepared using simple syrup as the suspending vehicle. The suspending agents included in the composition of the vehicles used in the current study made them suitable for the compounding of suspensions from pure drug powders. These vehicles are dye-free and have a light cherry flavour that may have a positive impact on the taste of the formulation and patients’ adherence with treatment.
As demonstrated in this study, suspensions of TMP and SMX should not be compounded from tablets because of physical incompatibility between these vehicles and (probably) the excipients in the tablets. Indeed, the suspension prepared from tablets in Oral Mix vehicle created excessive foam at the surface after shaking, which could lead to inconsistent dosing. In addition, although not observed in our study, solid caking at the bottom of bottles is hard to disperse and would lead to difficulty in resuspension by patients and inaccuracy in dosage.
Unfortunately, many public drug insurance plans (e.g., Régie de l’assurance maladie du Québec, Ontario Drug Benefit) do not reimburse preparations compounded from bulk drug powders because a Drug Identification Number (DIN) is required to submit a claim; as such, patients are limited to using only approved tablets or capsules. Nonetheless, we strongly recommend preparation of suspensions from bulk drug powder, given the unacceptable quality of preparations made from tablets. We also strongly believe it would be in the best public interest if TMP-SMX suspensions compounded from bulk powders were to be covered by public drug insurance plans in all provinces, as preparations from tablets result in unacceptable suspensions that can lead to dose inaccuracies and treatment failure.
CONCLUSION
Extemporaneous compounding of a liquid formulation of TMP-SMX is necessary for administration to patients incapable of swallowing tablets, including young children. This study has shown that compounding oral suspensions from bulk powder results in the only acceptable formulation, given that compounding from tablets produced unacceptable suspensions.
Suspensions of TMP and SMX (8 and 40 mg/mL, respectively) prepared from pure powder in Oral Mix or Oral Mix SF vehicle and stored in amber plastic bottles or amber syringes at 5°C or 25°C remained stable for 90 days.
References
1 Teva-Trimel tablets, Teva-Trimel DS tablets and Teva-Trimel oral suspension [product monograph]. Teva Canada Limited; 2018 Feb.
2 Caselli D, Petris MG, Rondelli R, Carraro F, Colombini A, Muggeo P, et al. Single-day trimethoprim/sulfamethoxazole prophylaxis for Pneumocystis pneumonia in children with cancer. J Pediatr. 2014;164(2): 389–392.e1.
Crossref
3 Hughes WT, Kuhn S, Chaudhary S, Feldman S, Verzosa M, Aur RJ, et al. Successful chemoprophylaxis for Pneumocystis carinii pneumonitis. N Engl J Med. 1977;297(26):1419–26.
Crossref
4 Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82(9):1052–9.
Crossref
5 Drug shortage report for Apo-Sulfatrim oral suspension. In: Drug shortages Canada [reporting website]. Health Canada; 2020 [cited 2020 Mar 12]. Available from: https://www.drugshortagescanada.ca/shortage/18991
6 Drug shortage report for Teva-Trimel. In: Drug shortages Canada [reporting website]. Health Canada; 2020 [cited 2020 Mar 12]. Available from: https://www.drugshortagescanada.ca/shortage/1591
7 Wu F, Shen L, Yang C, Chen C. Stability of extemporaneous suspensions of co-trimoxazole. Chinese Pharm J (Taipei). 1999;51(1):93–102.
8 Medisca’s oral bases [product information]. Medisca Pharmaceutique Inc; [cited 2020 Mar 12]. Available from: https://www.medisca.com/NDC_SPECS/MUS/2512/Downloads/Medisca%20Oral%20Bases%20Sell%20Sheet%20EN.pdf
9 LabSolutions data acquisition and processing theory guide. Instruction manual. Shimadzu Corporation; 2012.
Isabelle St-Jean
, MSc, is a Research Associate with the Faculty of Pharmacy, Université de Montréal, Montréal, Quebec
M Mihaela Friciu
, MSc, is a Research Associate with the Faculty of Pharmacy, Université de Montréal, Montréal, Quebec
Anaëlle Monfort
, BSc, is a PhD student with the Faculty of Pharmacy, Université de Montréal, Montréal, Quebec
Jessica MacMahon
, BPharm, MSc, is a Pharmacist with CHU Sainte-Justine, Montréal, Quebec
Jean-Marc Forest
, BPharm, MSc, is a Pharmacist with CHU Sainte-Justine, Montréal, Quebec
Scott Walker
, BPharm, MSc is a Staff Pharmacist with Sunnybrook Health Sciences Centre, Toronto, Ontario
Grégoire Leclair
, BPharm, PhD, is Full Professor with the Faculty of Pharmacy, Université de Montréal, Montréal, Quebec
Competing interests:
None declared. (
Return to Text
)
Address correspondence to: Grégoire Leclair, Faculté de pharmacie, Université de Montréal, PO Box 6128, Downtown Station, Montréal QC H3C 3J7,
email:
gregoire.leclair@umontreal.ca
(Return to Top)
Funding:
This study was funded by an unrestricted grant from Medisca. The company was not involved in the design or conduct of the study; the collection, management, or interpretation of the data; or the preparation, review, or approval of the manuscript. (
Return to Text
)
Canadian Journal of Hospital Pharmacy
, VOLUME
74
, NUMBER
4
,
Fall
2021


